|
 |
- Search
| Clin Exp Reprod Med > Volume 42(2); 2015 > Article |
Abstract
Objective
Artificial oocyte activation (AOA) is an effective method to avoid total fertilization failure in human in vitro fertilization-embryo transfer (IVF-ET) cycles. AOA performed using a calcium ionophore can induce calcium oscillation in oocytes and initiate the fertilization process. We evaluated the usefulness of AOA with a calcium ionophore in cases of total fertilization failure in previous cycles and in cases of severe male factor infertility patients with non-motile spermatozoa after pentoxifylline (PF) treatment.
Methods
The present study describes 29 intracytoplasmic sperm injection (ICSI)-AOA cycles involving male factor infertility at Cheil General Hospital from January 2006 to June 2013. Patients were divided into two groups (control, n=480; AOA, n=29) depending on whether or not AOA using a calcium ionophore (A23187) was performed after testicular sperm extraction-ICSI (TESE-ICSI). The AOA group was further split into subgroups according to sperm motility after PF treatment: i.e., motile sperm-injected (n=12) and non-motile sperm-injected (n=17) groups (total n=29 cycles).
Results
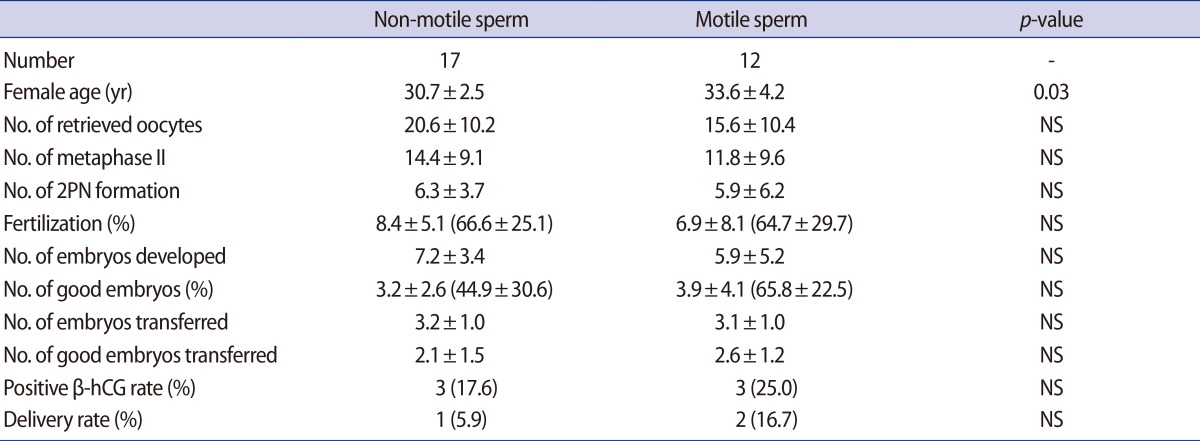
The good embryo rate (52.3% vs. 66.9%), pregnancy rate (20.7% vs. 52.1%), and delivery rate (10.3% vs. 40.8%) were lower in the PF/AOA group than in the control group. When evaluating the effects of restoration of sperm motility after PF treatment on clinical outcomes there was no difference in fertilization rate (66.6% vs. 64.7% in non-motile and motile sperm, respectively), pregnancy rate (17.6% vs. 33.3%), or delivery rate (5.9% vs. 16.7%) between the two groups.
Conventional in vitro fertilization (IVF) provides the possibility for fertilization in cases of infertility, but this technique is not effective for in cases of severe male factor infertility. In these cases, sperm cannot penetrate the zona pellucida, and intracytoplasmic sperm injection (ICSI) is applied to overcome this problem. ICSI has the potential to achieve fertilization even in cases of severe oligoasthenoteratozoospermia. There is a 60% to 70% fertilization rate in ICSI, but total fertilization failure occurs in 2% to 3% of ICSI cycles.
Several studies have proposed that fertilization failure after ICSI can be explained by defects in oocytes, sperm, or the ICSI procedure [1,2]. Lack of viability, abnormal chromatin status, inability of the sperm nucleus to decondense, and/or inability of sperm to activate oocytes are sperm defects that may account for failed fertilization after ICSI [3]. Investigation of unfertilized oocytes after ICSI reveals that these oocytes remain inactivated despite proper injection of spermatozoa [4,5]. More than 80% of unfertilized oocytes are arrested at the metaphase II (MII) stage, possibly due to failed oocyte activation [6].
During failed fertilization, the meiosis-to-mitosis transition is impaired, and thus fertilization failure is defined as failure of the M-G1 (growth phase-synthetic phase-G2 phase-mitosis phase) transition in MII oocytes [6]. Oocyte activation is characterized by a dramatic rise in intracellular calcium concentration, which in mammals takes the form of calcium oscillations driven by an elevation in inositol triphosphate (IP3) concentrations [7,8]. These oscillations have been proposed to be driven by a recently described phosphoinositide-specific phospholipase C, PLC-Z, which is a soluble sperm factor delivered to the egg following membrane fusion [9]. The induced calcium oscillation leads to resumption of meiosis, decondensation of the sperm nucleus, maternal RNA recruitment, formation of male and female pronuclei, initiation of DNA synthesis, and cleavage. Activation of the oocyte results in a cascade of events including extrusion of the second polar body, decondensation of a haploid set of chromosomes, formation of a nuclear membrane around the chromosomes and initiation of embryonic development [10,11].
Complete immotility of a sperm sample despite short culture in media is one cause of total fertilization failure in assisted reproductive technology (ART). Even before the introduction of ICSI, many investigators sought to prove that some phosphodiesterase inhibitors could improve or even induce sperm motility [12]. The most commonly used inducer of sperm motility is pentoxifylline (PF); in ART, the beneficial effects of PF on sperm motility and fertilization capacity in asthenozoospermia have been confirmed [13,14]. PF is a phosphodiesterase inhibitor of the methylxanthine group. It inhibits the breakdown of cyclic adenosine monophosphate, which is known to play a pivotal role in sperm motility [15].
In the IVF laboratory, artificial oocyte activation (AOA) and PF can be used in cases of male factor infertility. However in some cases, sperm motility is not restored despite PF treatment. In these cases, AOA can be carried out after PF-ICSI. There are few studies focused on PF treatment and AOA in cases where spermatozoa have no motility. Thus, the aim of this study was to evaluate the efficiency of PF and AOA on fertilization, cleavage rates, embryo development, and pregnancy rates after ICSI in cases of male factor infertility.
This study included patients who underwent AOA with a calcium ionophore after testicular sperm extraction-ICSI (TESE-ICSI) (AOA group; n=29) and patients who underwent TESE-ICSI without AOA (control group; n=480) at our center between January 2006 and June 2013. AOA group patients had a history of failed fertilization (n=3) or low fertilization (<45% of fertilization rate; n=26) in previous cycles. In this retrospective study, only ICSI cycles involving male factor infertility were included. Testicular sperm were evaluated according to World Health Organization criteria.
Ovarian stimulation was achieved using a gonadotropin-releasing hormone (GnRH) agonist (Lucrin, Abbott Korea Ltd., Seoul, Korea; Suprefact, Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany) and GnRH antagonist (Cetrotide, Merck Serono, Geneva, Switzerland or Orgalutran, Schering-Plough Organon, Oss, the Netherlands). Recombinant human chorionic gonadotropin (hCG) (Ovidrel, Merck Serono) was administered subcutaneously in a single 225 IU dose when the dominant follicle reached a maximum diameter of 18 mm or greater. Vaginal ultrasound-guided follicle puncture was performed 34-36 hours after hCG injection. Retrieved oocytes were cultured for several hours in germinal vesicle (GV) series medium (Vitrolife, Kungsbacka, Sweden) at 37℃ in an atmosphere of 6% CO2 under humidified conditions. All oocyte handling procedures were conducted on warm stages using conventional methods.
Testicular tissue specimens were obtained from non-obstructive azoospermia and obstructive azoospermia patients undergoing TESE. Testicular tissues were dissected and placed in a Petri dish filled with Ham's F-10 media with 0.4% human serum albumin (HSA, Sigma, St. Louis, MO, USA). For sperm extraction, the seminiferous tubules were washed two to three times in medium and squeezed with fine forceps under 10× magnification. The specimen was centrifuged at 1,460 rpm for 5 minutes and the pellet was suspended with G-GAMETE media (Vitrolife) and centrifuged again. The specimen was then incubated at 37℃ in 6% CO2 under humidified conditions.
After oocyte retrieval, oocytes were incubated in culture media (G-FERT, Vitrolife) with 10% HSA (Vitrolife) and treated in hyaluronidase (Sigma) in G-FERT medium. Oocytes were washed in fresh G-FERT and transferred to G-GAMETE under oil in a Falcon 1006 dish (Falcon, Franklin Lakes, NJ, USA) prepared for ICSI. For ICSI, individual oocytes were placed in droplets of buffered G-GAMETE medium. Sperm was placed in a central droplet of polyvinylpyrrolidone solution (PVP, SAGE, Trumbull, CT, USA) in a Falcon 1006 ICSI dish covered with warm mineral oil (SAGE oil, SAGE). Testicular sperm were assessed for motility; when there were no motile sperm, 5 mM PF (Sigma) was added to induce motility. Sperm injection was carried out on the heated stage (37℃) of an inverted microscope 38 hours after administration of the recombinant hCG trigger. After 30 minutes, post-ICSI oocytes were incubated in culture medium containing 10 uM calcium ionophore A23187 (Sigma) for 5 minutes at 37℃ and 6% CO2. The oocytes were then washed and placed in fertilization medium (G-FERT, Vitrolife) in the incubator under the same conditions.
Between 16 to 18 hours after ICSI, normal fertilization was assessed by the presence of two pronuclei. Embryo quality and fertilization were evaluated 24 or 48 hours after normal fertilization. The following parameters were analyzed: number of blastomeres, fragmentation percentage, variation in blastomere symmetry, and defects in cytoplasm. The assessment of fertilization and cleavage was performed by experienced embryologists.
Embryo transfer was performed on the second or third day of fertilization. One to four embryos were transferred, depending on the embryo quality, number of previous failed cycles and the age of the woman. Twelve days after oocyte collection, the blood concentration of β-hCG was measured (>5 mIU/mL).
We divided TESE-ICSI (ICSI using testicular sperm) patients into two groups and compared clinical outcomes between the AOA group (n=29) and the control group (n=480). Female age (31.6±3.6 vs. 33.1±4.2 years), the number of retrieved oocytes (18.7±10.1 vs. 14.3±8.4), and the fertilization rate (66.2%±25.8% vs. 74.3%±20.9%) were not significantly different between the two groups. The quality of embryos (52.3%±28.6% vs. 66.9%±29.3%, p<0.05) was significantly lower in the AOA group compared with the control group. The β-hCG positive (20.7% vs. 52.1%) and delivery (10.3% vs. 40.8%) rates were significantly lower in the AOA group than in the control group (Table 1).
In the 17 cases in the AOA group, sperm motility was not restored after PF treatment. These cases were assigned to undergo to ICSI with non-motile spermatozoa. Clinical outcomes were compared between the motile sperm ICSI and the non-motile sperm ICSI, and there were no differences between the two groups (Table 2).
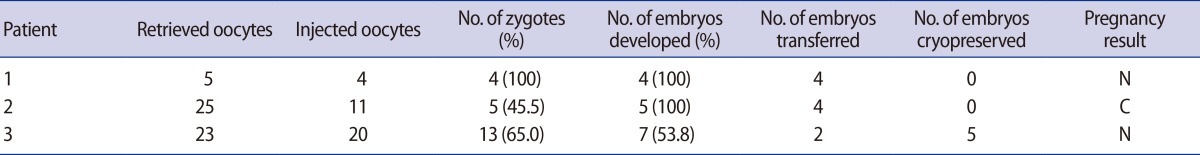
The clinical outcomes of the three cycles with total fertilization failure in previous cycles were analyzed after AOA-TESE; fertilization rates were 100%, 45.5%, and 65%, respectively. Moreover, embryo transfer was performed in all three cycles without cancellation, although clinical pregnancy was not achieved (Table 3).
IVF has been helpful in many cases of infertility, and ICSI has offered hope of achieving fertilization in cases of severe male infertility. However, a small number of oocytes remain unfertilized despite injection of spermatozoa. The low fertilization rate or total fertilization failure that occurs in some ICSI cases is due to non-activated oocytes. Several studies have reported success after previous fertilization failures by artificially activating oocytes [16]. In humans, the AOA protocol has included methods such as electrical stimulation and chemical treatments such as calcium ionophores. Calcium ionophore treatment is the most frequently used clinical method of oocyte activation. It has been suggested that the calcium oscillation pattern during oocyte activation may influence not only fertilization but also embryo development and, therefore, implantation [16]. Montag et al. [17] reported that AOA has particularly great potential in patients who show compromised fertilization rates below 30% after standard ICSI. AOA can promote a rise in intracellular calcium concentration, resulting in higher fertilization rates. The improved quality of embryos in several reports may be related to optimal oocyte activation, as optimized calcium oscillations can affect embryo development. Calcium oscillations may also participate in long-term embryonic events that go beyond its role as the stimulus for meiotic resumption [18]. The exact mechanism by which intracellular calcium influences embryonic development is not completely understood; however, it has been postulated that the calcium oscillation pattern may partly act through gene expression regulation [19,20].
In many cases, testicular sperm are immotile after biopsy, especially after the thawing of frozen testicular samples [21]. In the absence of motile sperm, biologists perform ICSI with immotile sperm; however, injection of immotile sperm results in decreased fertilization and pregnancy rates. Tug et al. [19] demonstrated the relationship between the percentage of non-motile sperm cells and total comet scores, providing evidence that DNA damage in spermatozoa and sperm motility parameters are negatively correlated. It has been reported that sperm with damaged DNA have a negative influence on pregnancy outcome [20].
PF treatment and AOA are generally performed to increase the fertilization rate in ICSI cases with a history of total fertilization failure in previous cycles or in cases of expectedly low fertilization rates due to the use of poor quality sperm. In our study, the good embryo rate, β-hCG positive rate and delivery rate were lower in the PF/AOA group than in the control group (Table 1). These differences in clinical outcomes may be influenced by sperm DNA integrity in PF/AOA cycles, a possibility that is supported by a previous report indicating that sperm motility may be correlated with its DNA integrity [22]. To analyze the effects of the restoration of sperm motility after PF treatment on clinical outcomes, the PF/AOA group was subdivided into two subgroups according to sperm motility: a motile sperm-injected and a non-motile sperm-injected group. There was no difference in fertilization rate, pregnancy rate and delivery rate between the two groups (Table 2). The AOA group also included three cases with a history of fertilization failure in a previous cycle. Two of these cases did not achieve pregnancy while one case achieved a chemical pregnancy (Table 3). However, in cases of total fertilization failure or lower fertilization rate in the previous cycle, AOA treatment prevented cancellation of the embryo transfer cycles owing to fertilization failure.
In conclusion, AOA may be useful in selected patients who have a low fertilization rate or total fertilization failure. Our data suggests that oocyte activation is a useful method to ensure fertilization in a TESE-ICSI cycle regardless of the restoration of sperm motility after PF treatment.
References
1. Farhi J, Ben-Haroush A, Dresler H, Pinkas H, Sapir O, Fisch B. Male factor infertility, low fertilisation rate following ICSI and low number of high-quality embryos are associated with high order recurrent implantation failure in young IVF patients. Acta Obstet Gynecol Scand 2008;87:76-80.PMID: 17963052.


3. Nasr-Esfahani MH, Deemeh MR, Tavalaee M. Artificial oocyte activation and intracytoplasmic sperm injection. Fertil Steril 2010;94:520-526.PMID: 19393997.


4. Wall MB, Marks K, Smith TA, Gearon CM, Muggleton-Harris AL. Cytogenetic and fluorescent in-situ hybridization chromosomal studies on in-vitro fertilized and intracytoplasmic sperm injected 'failed-fertilized' human oocytes. Hum Reprod 1996;11:2230-2238.PMID: 8943535.


5. Dubey AK, Penzias AS, Emmi AE, Layman LC, Reindollar RH, Ducibella T. Failed fertilization after intracytoplasmic sperm injection: the extent of paternal and maternal chromatin decondensation. Fertil Steril 1997;68:714-717.PMID: 9341616.


6. Buffone MG, Schindler K, Schultz RM. Overexpression of CDC14B causes mitotic arrest and inhibits zygotic genome activation in mouse preimplantation embryos. Cell Cycle 2009;8:3904-3913.PMID: 19923902.



7. Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol 1999;211:157-176.PMID: 10395780.


8. Rice A, Parrington J, Jones KT, Swann K. Mammalian sperm contain a Ca(2+)-sensitive phospholipase C activity that can generate InsP(3) from PIP(2) associated with intracellular organelles. Dev Biol 2000;228:125-135.PMID: 11087632.


9. Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, et al. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development 2002;129:3533-3544.PMID: 12117804.

10. Tosti E, Boni R. Electrical events during gamete maturation and fertilization in animals and humans. Hum Reprod Update 2004;10:53-65.PMID: 15005464.


11. McLay DW, Carroll J, Clarke HJ. The ability to develop an activity that transfers histones onto sperm chromatin is acquired with meiotic competence during oocyte growth. Dev Biol 2002;241:195-206.PMID: 11784105.


12. Aparicio NJ, de Turner EA, Schwarzstein L, Turner D. Effect of the phosphodiesterase inhibitor Pentoxyfylline on human sperm motility. Andrologia 1980;12:49-54.PMID: 7377553.


13. Morales P, Llanos M, Yovich JL, Cummins JM, Vigil P. Pentoxifylline increases sperm penetration into zona-free hamster oocytes without increasing the acrosome reaction. Andrologia 1993;25:359-362.PMID: 8279710.


14. Kovacic B, Vlaisavljevic V, Reljic M. Clinical use of pentoxifylline for activation of immotile testicular sperm before ICSI in patients with azoospermia. J Androl 2006;27:45-52.PMID: 16400077.


15. Tash JS, Means AR. Cyclic adenosine 3',5' monophosphate, calcium and protein phosphorylation in flagellar motility. Biol Reprod 1983;28:75-104.PMID: 6299416.


16. Ozil JP, Banrezes B, Toth S, Pan H, Schultz RM. Ca2+ oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev Biol 2006;300:534-544.PMID: 16996050.


17. Montag M, Koster M, van der Ven K, Bohlen U, van der Ven H. The benefit of artificial oocyte activation is dependent on the fertilization rate in a previous treatment cycle. Reprod Biomed Online 2012;24:521-526.PMID: 22417664.


18. Borges E Jr, de Almeida Ferreira Braga DP, de Sousa Bonetti TC, Iaconelli A Jr, Franco JG Jr. Artificial oocyte activation with calcium ionophore A23187 in intracytoplasmic sperm injection cycles using surgically retrieved spermatozoa. Fertil Steril 2009;92:131-136.PMID: 18692786.


19. Tug N, Sandal S, Ozelgun B, Yilmaz B. Correlation of spermiogram profiles with DNA damage in sperm cells of infertile men: a comet assay study. Gynecol Endocrinol 2011;27:49-54.PMID: 20504093.


20. Park YS, Kim MK, Lee SH, Cho JW, Song IO, Seo JT. Efficacy of testicular sperm chromatin condensation assay using aniline blue-eosin staining in the IVF-ET cycle. Clin Exp Reprod Med 2011;38:142-147.PMID: 22384433.



21. Bachtell NE, Conaghan J, Turek PJ. The relative viability of human spermatozoa from the vas deferens, epididymis and testis before and after cryopreservation. Hum Reprod 1999;14:3048-3051.PMID: 10601095.


22. Chi HJ, Chung DY, Choi SY, Kim JH, Kim GY, Lee JS, et al. Integrity of human sperm DNA assessed by the neutral comet assay and its relationship to semen parameters and clinical outcomes for the IVF-ET program. Clin Exp Reprod Med 2011;38:10-17.PMID: 22384412.