|
 |
- Search
| Clin Exp Reprod Med > Volume 40(3); 2013 > Article |
Abstract
Objective
To evaluate the effect of the addition of estradiol to luteal progesterone supplementation in GnRH antagonist cycles for infertile patients undergoing IVF/ICSI.
Methods
One hundred and ten infertile patients, aged 28 to 39 years, were recruited for this prospective randomized study. They were randomly assigned to receive vaginal progesterone gel (Crinone) along with 4 mg estradiol valerate (group 1, n=55) or only Crinone (group 2, n=55) for luteal support. A GnRH antagonist multiple dose protocol using recombinant human FSH was used for controlled ovarian stimulation (COS) in all of the subjects. The COS results and pregnancy outcomes of the two groups were compared.
Results
Group 1 and 2 were comparable with respect to the patient characteristics. The COS and IVF results were also comparable between the two groups. There were no differences in the clinical pregnancy rate (PR) and multiple PR between the two groups. However, the embryo implantation rate were significantly higher in group 1 than that in group 2 (22.2% vs. 13.3%, p=0.035). The incidence of luteal vaginal bleeding (LVB) was significantly lower in group 1 (7.4% vs. 27.8%, p=0.010).
Luteal phase supplementation after controlled ovarian stimulation (COS) for IVF-ET has been current practice, because stimulated IVF cycles are associated with luteal phase defect (LPD) due to very low LH concentration after COS [1].
Luteal supplementation is important for successful embryo implantation after COS for IVF. Progesterone supplementation in the luteal phase after COS is widely accepted, and the role of progesterone in luteal support in COS cycles is well established. However, it has been shown that mid-luteal estradiol levels decrease under progesterone supplementation alone. This might be associated with a decrease in pregnancy rates [2]. In addition, luteal vaginal bleeding (LVB) can develop more frequnetly in patients supplemented with progesterone vaginal gel, or vaginal suppositories containing micronized progesterone compared with those who receive intramuscular progesterone [3], although it is very easy and convenient to use progesterone vaginal gel. The importance of estradiol levels during the luteal phase or the addition of luteal estradiol to progesterone supplementation for luteal support in the IVF cycle is controversial [4].
This prospective, randomized study was performed to evaluate the effect of the addition of estradiol to luteal vaginal suppositories containing micronized progesterone supplementation in GnRH antagonist cycles for infertile patients undergoing IVF/ICSI.
This prospective randomized study was performed at a university-based infertility clinic at Asan Medical Center, Seoul, Korea. The study population consisted of 110 infertile patients who had undergone 110 IVF cycles. Patients were randomized to receive progesterone vaginal gel (Crinone 8%, Merck-Serono SA, Geneva, Switzerland), progesterone vaginal gel along with estradiol valerate (Progynova, Bayer-Schering, Berlin, Germany) (group 1) or only progesterone vaginal gel (group 2). They were in good health with normal thyroid, hepatic and renal functioning. The Institutional Review Board of our center approved the study (2006-0446) and all of the patients provided written informed consent.
The GnRH antagonist multiple dose protocol (MDP) using recombinant human follicle stimulating hormone FSH (rhFSH) was used for COS in all of the subjects. On cycle day 3, ovarian stimulation was commenced using rhFSH (Gonal-F, Merck-Serono SA) of 150 to 225 IU/day after establishing ovarian and uterine quiescence using vaginal ultrasound. The rhFSH dose was adjusted according to the ovarian response, every 3 to 4 days. GnRH antagonist (Cetrotide, 0.25 mg; Merck-Serono SA) was started when the leading follicle reached an average of 14 mm in diameter, and was continued daily until the day of hCG administration. Recombinant hCG (rhCG, Ovidrel, Merck-Serono SA) of 250 ┬Ąg was injected to induce follicular maturation when one or more follicles reached a mean diameter of Ōēź18 mm. Oocyte retrieval was performed 35 to 36 hours after hCG injection and one to three embryos were transferred into the uterus on the third day after oocyte retrieval. For group 1, 90 mg of vaginal progesterone gel (Crinone 8%) once daily and estradiol valerate orally 4 mg daily were administrated for luteal phase support from the day of oocyte retrieval. For group 2, only 90 mg of vaginal progesterone gel was administered during the same period. The serum level of ╬▓-hCG was measured 11 days after embryo transfer (ET). On the day of the first pregnancy test, patients were asked by the clinician in our fertility clinic if they had experienced any bleeding. Clinical pregnancy was defined as an increased serum ╬▓-hCG concentration, as measured by radioimmunoassay using a hCG MAIAclone kit (Serono Diagnostics, Woking, Surrey, UK) with interassay and intraassay variances of <10% and 5%, respectively, and transvaginal ultrasonographic evidence of a gestational sac. Estradiol valerate supplementation was discontinued on the day of the first pregnancy test, 11 days after ET. All of the patients who had increased serum ╬▓-hCG were administered vaginal progesterone gel continuously until 10 weeks of gestation.
The mean value was expressed as the mean┬▒SD. The Student's t-test was used to compare the mean values between the two groups. The chi-squared test and Fisher's exact test were used for the comparison of fractions, where applicable. Statistical significance was defined as p<0.05. All analyses were performed using the SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA).
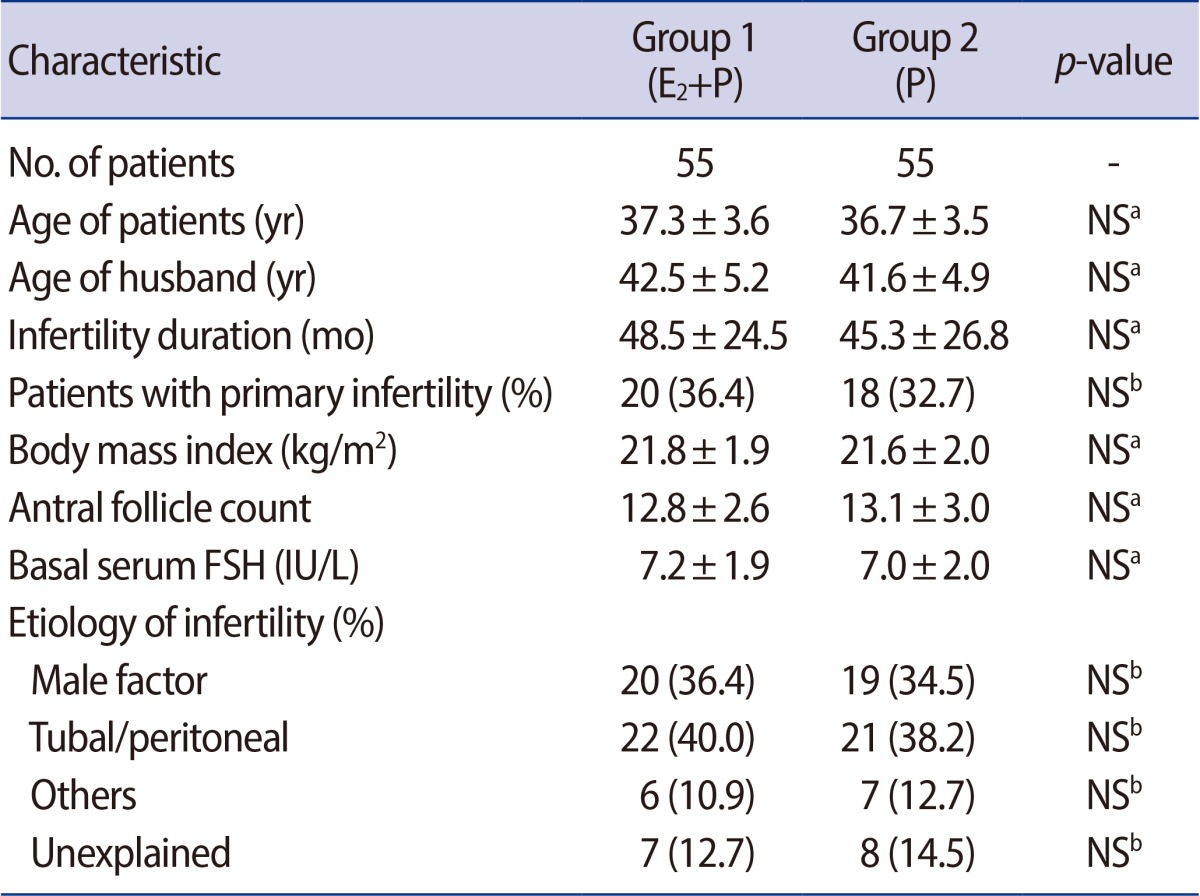
Group 1 and 2 were comparable with respect to the patient's characteristics such as the age of patients, infertility duration, the proportion of nullipara, body mass index (BMI), antral follicle count (AFC) and basal serum FSH level (Table 1).
Each group 1 and 2 consisted of 55 cycles initiated corresponding to 55 patients. In group 1, 1 out of 55 cycles initiated (1.8%) was cancelled before ET, because no oocytes were obtained despite a follicular aspiration for oocyte retrieval. In group 2, 1 out of 55 cycles initiated (1.8%) was cancelled after oocyte retrieval due to a high risk of ovarian hyperstimulation syndrome (OHSS). There was no significant difference in the cycle cancellation rate between the two groups. Table 2 presents the comparison of COS results and IVF outcomes between groups 1 and 2. The two groups were similar in total the days and dose of rhFSH required for COS. There were no significant differences between the two groups with respect to the numbers of oocytes retrieved, mature oocytes, fertilized oocytes, grade I or II embryos, embryos transferred and embryos frozen (Table 2). There were also no differences in the clinical pregnancy rate (PR) and multiple PR between the two groups (Table 2). However, the embryo implantation rate was significantly higher in group 1, 26.0% (38/146) compared with 15.8% (24/152) in group 2 (p=0.033) (Table 2). The incidence of luteal vaginal bleeding (LVB) was significantly lower in group 1 than in group 2 (5.6% vs. 24.1% respectively, p=0.013) (Table 2). In group 1, LVB occurred with equal frequency in the pregnant and nonpregnant subgroups (3.8% vs. 7.1%, respectively). There were no differences in the pregnancy rate between patients who experienced LVB and those who did not (33.3% [1/3] vs. 49.0% [25/51], respectively). However, in group 2 supplemented with only Crinone, the incidence of LVB was significantly higher in the nonpregnant subgroup, at 35.3% (12/34) compared with 5.0% (1/20) in the pregnant subgroup (p=0.019). Those who experienced LVB had significantly lower pregnancy rates than those who did not experience LVB (7.7% [1/13] vs. 46.3% [19/41] respectively, p=0.019).
COS has contributed to improving assisted reproductive technology (ART) outcomes. However, COS frequently results in luteal phase defect (LPD). The luteal function could be attributed to COS, resultant altered hormone levels and the process of oocyte retrieval. The elevation of serum estradiol to supraphysiologic levels by COS was prone to alter endometrial receptivity by causing an imbalance of the estradiol/progesterone ratio. Follicular fluid aspiration for oocyte retrieval may disrupt and reduce the number of granulosa cells undergoing luteinization, thereby diminishing the corpus luteal function and reducing progesterone levels. Luteal function can be suppressed by the direct effect of GnRH agonist on the corpus luteum in a GnRH agonist long protocol [5]. Applying GnRH antagonist co-treatment in IVF cycles has also shown that luteolysis is initiated prematurely, resulting in a significant reduction in the length of the luteal phase [5]. In these conditions, LPD can be overcome by supplementation of hCG or progesterone, a concept referred to as luteal phase support, and this modality has been the standard for luteal phase support since late the 1980s [6]. A recent meta-analysis demonstrated that the effect of hCG is comparable to progesterone for luteal phase support with respect to clinical PR [6]. Nevertheless, progesterone is often favored, because hCG is closely related to the development of OHSS. Various preparations of progesterone including oral, intramuscular (IM) and vaginal forms are currently available. A recent meta-analysis investigating possible differences in ART outcomes between the different progesterone preparations have shown that IM and vaginal progesterone are equally effective for luteal phase support [7]. IM progesterone is often associated with many side effects such as painful injection, skin rash, urticaria and inflammatory reactions. Therefore, vaginal progesterone is frequently favored. However, a few studies have reported a higher incidence of LVB in patients supplemented with the vaginal progesterone gel, Crinone compared with those supplemented with IM progesterone [3]. The clinical significance of LVB remains unclear. In patients who received only Crinone in our study, LVB occurred more frequently in the nonpregnant subgroup than in the pregnant subgroup (35.3% vs. 5.0%, respectively). In addition, in patients who received Crinone with estradiol in our study, LVB occurred more frequently in the nonpregnant subgroup than in the pregnant subgroup (7.1% vs. 3.8%, respectively). In a recent study, Yanushpolsky et al. [8] reported the incidence of LVB in pregnant patients who were supplemented with estradiol was significantly lower than in those who were supplemented with only progesterone (10% vs. 23.9%, respectively). It is unknown whether LVB is the cause or result of embryo implantation failure. However, LVB may be an ominous sign of implantation failure and be disquieting to both patients and physicians. Therefore, an effort to reduce LVB is needed. Actually, in the present study, the addition of estradiol valerate to luteal Crinone supplementation in GnRH antagonist cycles significantly reduced the incidence of LVB, while also increasing the embryo implantation rate.
Today evidence is mounting that COS is associated with the occurrence of an abnormal luteal phase with characteristic features of decreased production of estradiol and progesterone levels and significantly reduced luteal phase length. Therefore, the addition of estradiol to progesterone supplementation may be more effective for luteal phase support compared with progesterone supplementation alone. Several clinical trials have investigated the effect of adding estradiol to progesterone during luteal phase support in the ART cycle. These studies differed in the type of COS protocol, dose and type of estradiol, and type of progesterone used. A recent randomized controlled trial (RCT) evaluating the effect of adding oral estradiol to luteal progesterone in GnRH agonist down-regulation cycles showed no benefits of adding estradiol. In this recent RCT, the serum progesterone concentrations on day 7, 10, and 12 after ET were similar in the luteal estradiol addition group and luteal progesterone only group [9]. On the other hand, Farhi et al. [10] reported that estradiol supplementation during the luteal phase improved the PR and implantation rates in patients who were treated with a long GnRH agonist protocol for COS. In addition, a RCT by Ghanem et al. [11] demonstrated that luteal estradiol addition in long GnRH agonist protocol cycles resulted in a significantly higher clinical PR and implantation rate in patients who underwent ICSI. In our present study, only GnRH antagonist MDP cycles were included. Although clinical PR per cycle initiated was higher in the estradiol addition group in comparison to the Crinone only group, this difference did not achieve statistical significance. However, the addition of estradiol to luteal Crinone supplementation significantly reduced the incidence of LVB and increased the embryo implantation rate. These results support that estradiol has an active role in the implantation process and reduced estradiol levels during the luteal phase leading to a reduced chance of conception.
In conclusion, LVB during Crinone supplementing the luteal phase may be related to the embryo implantation failure in GnRH antagonist protocol IVF/ICSI cycles and therefore an effort to reduce LVB may be required. Furthermore, the addition of estradiol to luteal Crinone supplementation in GnRH antagonist cycles may reduce the incidence of LVB and increase the implantation rate in infertile patients undergoing IVF/ICSI.
References
1. Tavaniotou A, Albano C, Smitz J, Devroey P. Comparison of LH concentrations in the early and mid-luteal phase in IVF cycles after treatment with HMG alone or in association with the GnRH antagonist Cetrorelix. Hum Reprod 2001;16:663-667.PMID: 11278214.


2. Sharara FI, McClamrock HD. Ratio of oestradiol concentration on the day of human chorionic gonadotrophin administration to mid-luteal oestradiol concentration is predictive of in-vitro fertilization outcome. Hum Reprod 1999;14:2777-2782.PMID: 10548621.


3. Propst AM, Hill JA, Ginsburg ES, Hurwitz S, Politch J, Yanushpolsky EH. A randomized study comparing Crinone 8% and intramuscular progesterone supplementation in in vitro fertilization-embryo transfer cycles. Fertil Steril 2001;76:1144-1149.PMID: 11730742.


4. Engmann L, DiLuigi A, Schmidt D, Benadiva C, Maier D, Nulsen J. The effect of luteal phase vaginal estradiol supplementation on the success of in vitro fertilization treatment: a prospective randomized study. Fertil Steril 2008;89:554-561.PMID: 17678651.


5. Fauser BC, Devroey P. Reproductive biology and IVF: ovarian stimulation and luteal phase consequences. Trends Endocrinol Metab 2003;14:236-242.PMID: 12826330.


6. Pritts EA, Atwood AK. Luteal phase support in infertility treatment: a meta-analysis of the randomized trials. Hum Reprod 2002;17:2287-2299.PMID: 12202415.


7. van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev 2011;(10): CD009154PMID: 21975790.


8. Yanushpolsky E, Hurwitz S, Greenberg L, Racowsky C, Hornstein M. Patterns of luteal phase bleeding in in vitro fertilization cycles supplemented with Crinone vaginal gel and with intramuscular progesterone--impact of luteal estrogen: prospective, randomized study and post hoc analysis. Fertil Steril 2011;95:617-620.PMID: 20537624.


9. Moini A, Zadeh Modarress S, Amirchaghmaghi E, Mirghavam N, Khafri S, Reza Akhoond M, et al. The effect of adding oral oestradiol to progesterone as luteal phase support in ART cycles: a randomized controlled study. Arch Med Sci 2011;7:112-116.PMID: 22291742.



10. Farhi J, Weissman A, Steinfeld Z, Shorer M, Nahum H, Levran D. Estradiol supplementation during the luteal phase may improve the pregnancy rate in patients undergoing in vitro fertilization-embryo transfer cycles. Fertil Steril 2000;73:761-766.PMID: 10731538.


11. Ghanem ME, Sadek EE, Elboghdady LA, Helal AS, Gamal A, Eldiasty A, et al. The effect of luteal phase support protocol on cycle outcome and luteal phase hormone profile in long agonist protocol intracytoplasmic sperm injection cycles: a randomized clinical trial. Fertil Steril 2009;92:486-493.PMID: 19464001.


Table┬Ā2
Comparison of controlled ovarian stimulation results and IVF outcome
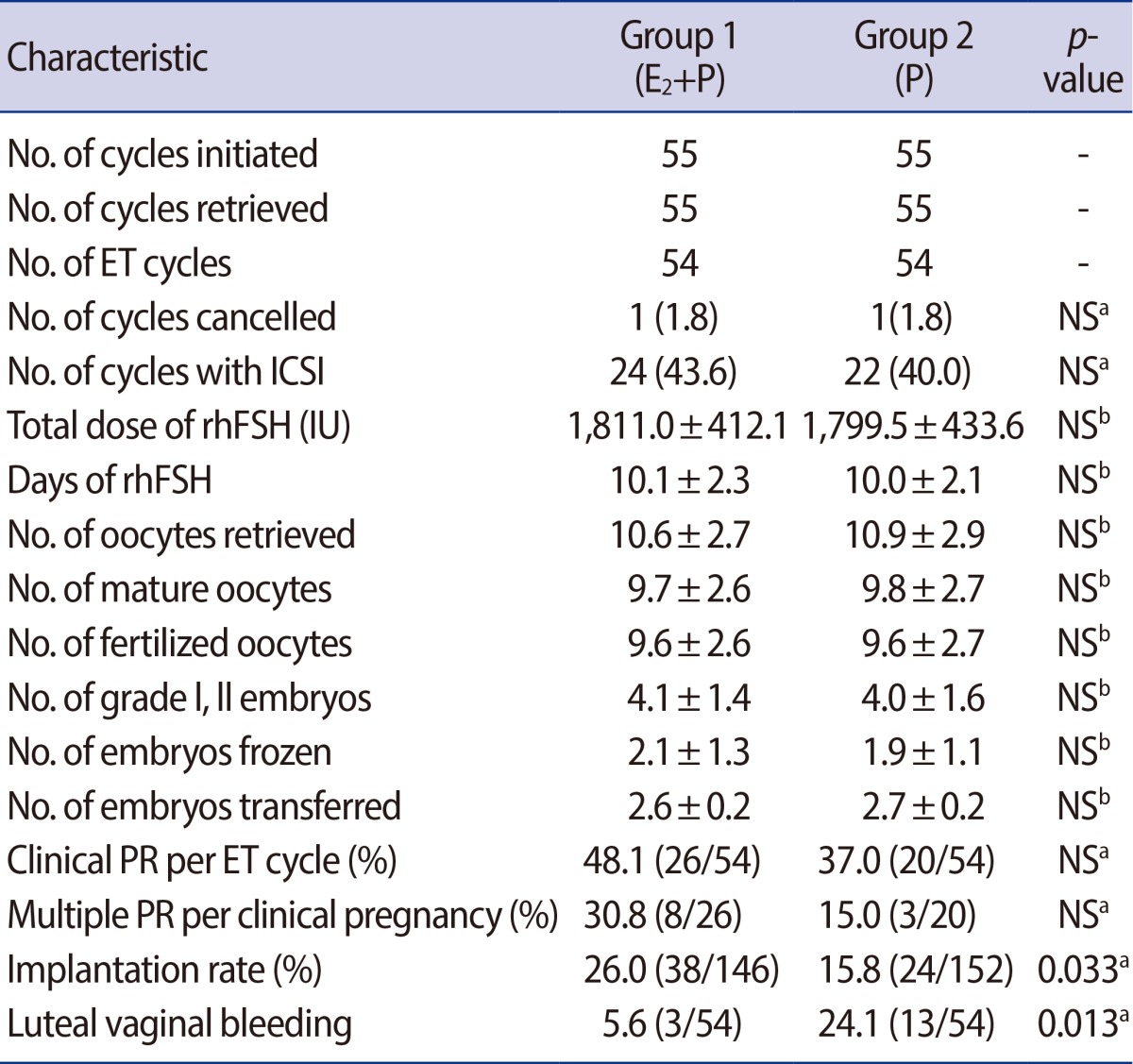
Values are presented as mean┬▒SD or number (%).
IVF, in vitro fertilization; E2, estradiol; P, progesterone; ET, embryo transfer; ICSI, intracytoplasmic sperm injection; rhFSH, recombinant human follicle stimulating hormone; PR, pregnancy rate; NS, not significant.
aChi-squared test or Fisher's exact test; bStudent's t-test.