Introduction
Physiologic levels of reactive oxygen species (ROS) are essential for gamete function and embryo development.
In vivo, the female reproductive tract produces antioxidants to maintain an optimal level of ROS [
1]. In contrast, in assisted reproductive technology (ART), the environment surrounding gametes and embryos lacks this natural antioxidant system. Furthermore, the
in vitro fertilization (IVF) process and culture environment can create an incidental build-up of ROS. When ROS production exceeds the cellular defense system, oxidative stress occurs, causing oxidative damage to lipids, proteins, and DNA, resulting in defective gametes and poor-quality embryos [
2]. To maintain optimal ROS levels during the IVF process and prevent oxidative stress development, excess ROS must be continuously counteracted. Several antioxidant agents have been used to supplement media with the aim of improving
in vitro embryo production, such as acetyl-L-carnitine, N-acetyl-L-cysteine, ╬▒-lipoic acid, selenium, vitamin E, and vitamin C [
3-
7].
In this study, we focused on astaxanthin (AST), a powerful antioxidant of the xanthophyll carotenoid group. AST is a red, lipid-soluble pigment extracted mainly from
Haematococcus pluvialis algae [
8]. It has 10 times more active free radical scavengers than other carotenoids (zeaxanthin, lutein, canthaxanthin, ╬▓-carotene) and 100 times more than ╬▒-tocopherol [
9]. AST has been widely utilized in dietary supplements that claim to possess anti-cancer, anti-diabetes, anti-aging, and anti-inflammatory properties. However, very few studies have been done on the use of AST supplements in laboratory media. We investigated the effect of AST supplementation in fertilization medium and/or embryo culture medium according to the two phases of the IVF process (gamete fertilization and embryo development), in order to observe the effect of AST on the fertilization rate, the blastocyst formation rate, and the blastocyst cell numbers.
Methods
1. Animals
The Animal Ethics Committee of the Faculty of Medicine, Chiang Mai University approved the use of mice in our study (protocol No. 47/2020). The investigators were competent in the use and care of animals for research and were certified by the Institute of Animals for Scientific Purposes Development, National Research Council of Thailand.
Male and female mice bred at the Institute of Cancer Research were obtained from the National Animal Institute, Mahidol University, Bangkok, Thailand. All mice were kept in an optimized environment in the animal husbandry unit at the Faculty of Medicine, Chiang Mai University. The room had adequate ventilation, was kept at a temperature of 25┬░C┬▒2┬░C and 60%ŌĆō70% humidity, and had controlled 12-hour light/dark cycles. The mice were left undisturbed for 7 days to minimize the transportation stress effect. The international and national guidelines for ethical conduct in the care and use of animals for research were followed.
2. Collection of cumulus-oocyte complexes
For superovulation, 5- to 9-week-old female mice were injected intraperitoneally (IP) with 10 units of pregnant mareŌĆÖs serum gonadotropin (Sigma, St. Louis, MO, USA). Forty-eight hours later, the mice received IP injections of 10 units of human chorionic gonadotropin (Pregnyl, Merck, NJ, USA). Sixteen hours after the second injection, the mice were killed by dislocation of the cervical vertebrae. The peritoneal cavity was exposed and the two oviducts were aseptically removed and placed in EarleŌĆÖs Balanced Salts Solution (Biological Industries, Kibbutz Beit Haemek, Israel), containing 0.5% bovine serum albumin (Sigma). Cumulus-oocyte complexes (COCs) were removed from the oviduct and separated into four groups for the experiment. COCs from each mouse were distributed to all four experimental groups. The goal was to reduce the potential variations among oocytes from different mice.
3. Experimental design
AST (Sigma; extracted from H. pluvialis algae) was dissolved in dimethyl sulfoxide at a concentration of 0.0005%. In preliminary trials, different concentrations of AST (1, 2.5, 5, 10, and 20 ┬ĄM) were examined. The addition of 5 ┬ĄM AST provided the best blastocyst formation rates in mouse oocytes. Therefore, a 5 ┬ĄM concentration of AST was used in this study. The basic mediums used in this study were fertilization medium (G-IVF; Vitrolife, Sydney, Australia), cleavage medium (G1-plus; Vitrolife), and blastocyst medium (G2-plus; Vitrolife).
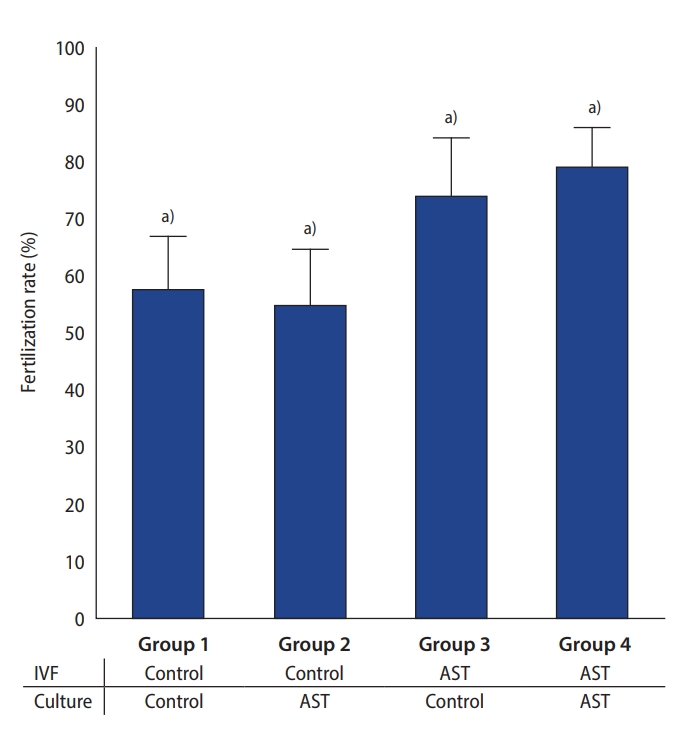
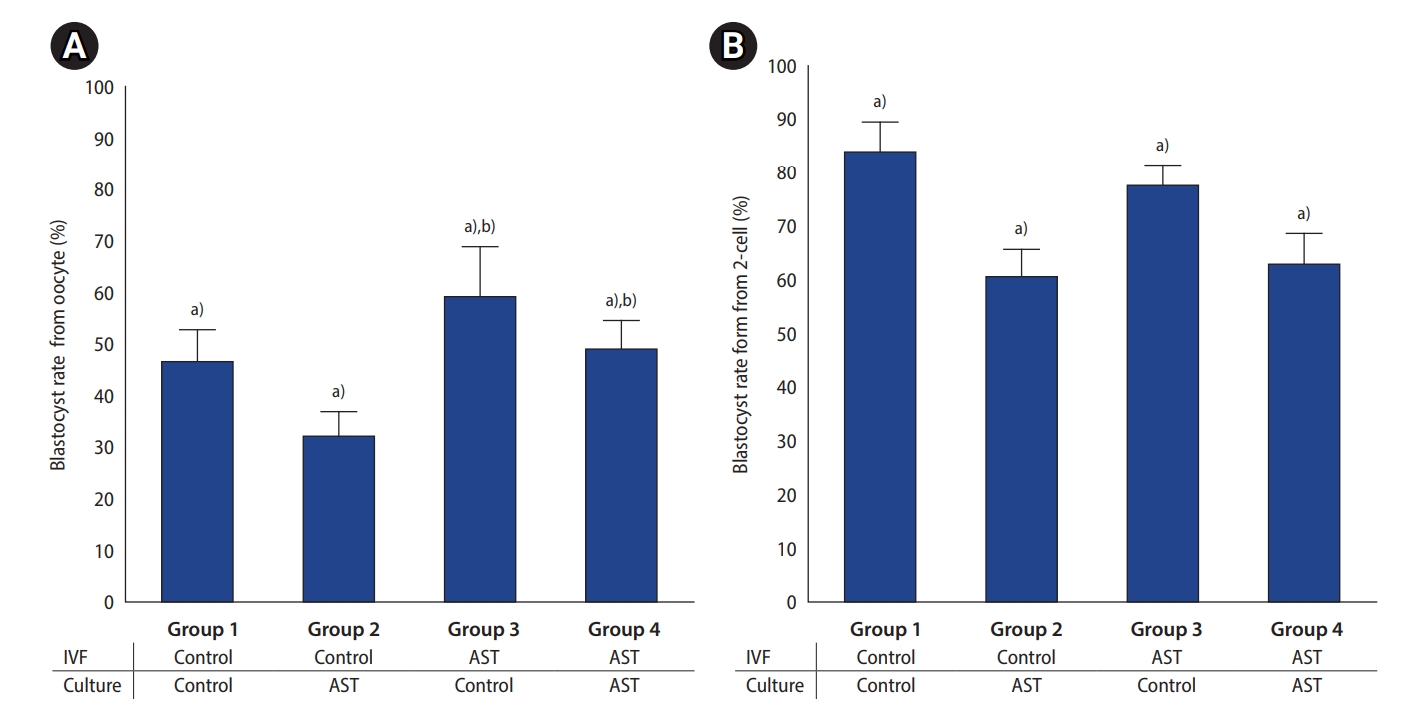
To assess the effect of AST supplementation in the gamete fertilization medium and/or embryo culture medium, four experimental groups were studied: group 1: no AST supplement was added to the fertilization medium or to the culture medium; group 2: the culture medium was supplemented with 5 ┬ĄM AST; group 3: the fertilization medium was supplemented with 5 ┬ĄM AST; group 4: both fertilization and culture mediums were supplemented with 5 ┬ĄM AST.
4. In vitro fertilization and embryo culture
To obtain sperm, 10- to 12-week-old male mice were killed by dislocation of the cervical vertebrae. The epididymis was excised, the tissue was nicked to release the sperm, and the sperm were placed in 1 mL of G-IVF (Vitrolife). The suspensions were allowed to capacitate for 30 minutes in the incubator at 37┬░C, 6% CO
2, 5% O
2, and 89% N
2. The spermatozoa were transferred to each experimental group of COCs in 50 ┬ĄL drops of G-IVF (Vitrolife) under mineral oil (Irvine Scientific, Santa Ana, CA, USA) with or without AST, as assigned, for insemination at a final motile sperm concentration of 2.5 ├Ś10
5/mL. Two hours later, fertilized oocytes were transferred to the culture in groups of 10 in 10 ┬ĄL drops of G1-plus (Vitrolife) under mineral oil (Irvine Scientific) with or without AST, as assigned. The fertilization rate was determined the next day by counting the number of two-cell embryos. Seventy-two hours post-insemination, the embryos were transferred to G2-plus (Vitrolife) under mineral oil (Irvine Scientific) with or without AST, as obtained in G1-plus, and cultured in similar conditions for 48 hours. Embryo development was assessed daily under an inverted microscope until reaching 120 hours of embryo culture growth. The mouse blastocysts were classified as early, partial, full, expanding, hatching and hatched blastocysts, using the human blastocyst development criteria proposed by Gardner et al. [
10].
5. Differential staining of the inner cell mass and trophectoderm cells
At 120 hours of embryo culture growth, differential staining was performed on all expanding, hatching, and hatched blastocysts, using the protocol described by Pampfer et al. [
11]. Briefly, the blastocyst with intact zona was placed in a 0.5% pronase solution (Sigma P8811) for 10 minutes to remove the zona pellucida. The zona-free blastocysts were then washed in calcium and magnesium-free phosphate-buffered saline (PBS; Gibco, Waltham, MA, USA). Washed blastocysts were exposed to rabbit anti-mouse antibody (Sigma M5774; concentration 1:50) for 30 minutes at 37┬░C, then washed in calcium and magnesium-free PBS, and transferred into a solution containing: (1) guinea pig complement serum (Sigma S1639; concentration 1:4), (2) 10 ╬╝g/mL Hoechst 33342 (Sigma H1399), and (3) 20 ╬╝g/mL propidium iodide (Sigma P4170) for 30 minutes at 37┬░C. The blastocysts were washed, placed on a glass slide, and allowed to air dry. The slides were covered with coverslips and mounted with glycerol. The cell numbers were counted using a fluorescence microscope (Nikon E600) with an excitation filter of 330-385 nm and a barrier filter of 400 nm, and then analyzed using LUCIA Cytogenetics FISH software (Laboratory Imaging, Prague, Czech Republic). The inner cell mass (ICM) nuclei were stained blue with Hoechst, while trophectoderm (TE) nuclei were stained red with propidium iodide.
6. Statistical analysis
The statistical analysis was performed using SPSS ver. 16 (SPSS Inc., Chicago, IL, USA). The fertilization rates and blastocyst formation rates among the four experimental groups were compared using the chi-square test. The mean numbers of ICM, TE, and total cells, as well as the ICM-to-TE ratio, were compared by one-way analysis of variance (ANOVA) when the data distribution was normal, or the Kruskal-Wallis test when normality could not be confirmed. P-values <0.05 were considered to indicate statistical significance.
Discussion
Oxidative stress had a toxic effect on the gametes and embryos during IVF and embryo development. The high ROS levels resulted in impaired oocyte quality, loss of sperm membrane integrity, sperm DNA fragmentation, diminished embryo quality, and reduced blastocyst development rates [
12]. The exogenous conditions causing this supraphysiologic level of ROS included high oxygen concentration, culture medium composition, energy source, changes in pH and temperature, visible light exposure, and the manipulation of gametes/embryos [
13]. Strategies to overcome the oxidative stress of the ART process by reducing incidental production of ROS include: (1) decreasing oxygen tension to 5% to more closely match the oxygen concentration of the oviduct and uterus (2% to 8%), (2) the use of light filters, (3) controlling the stability of pH and temperature, (4) the addition of metal chelators in the culture medium, and (5) adjusting techniques for handling, preparing, and manipulating the gametes/embryos during the ART procedure [
13]. Another method to address incidental ROS production was increasing the free radical scavenger to detoxify the excess ROS, by adding antioxidants to the IVF process and culture system [
3].
An antioxidant is a scavenging enzyme or molecule that converts ROS to water. Antioxidants are classified as enzymatic or non-enzymatic. Enzymatic antioxidants include superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase. Non-enzymatic antioxidants are also known as synthetic or dietary antioxidants (e.g., vitamin C, vitamin E, ╬▓-carotene, selenium, zinc, taurine, and glutathione) [
14]. AST is a carotenoid nutrient with a unique molecular structure containing conjugated double bonds, and hydroxyl (ŌĆōOH) and keto (C=O) groups, which contribute to its high antioxidant properties [
15]. AST spans itself across the cell membrane by using its polar end groups to overlap the polar boundary zones of the membrane. In this way, it scavenges free radicals both within the membraneŌĆÖs hydrophobic interior and along its hydrophilic boundaries [
16]. AST has been widely used as a dietary supplement due to its anti-inflammatory, antioxidant, anti-aging, anti-diabetes, and anti-cancer properties. Utilization of AST in the reproductive field has been limited, especially as an
in vitro supplement in the IVF medium during the ART process. Previous studies found that AST supplementation in the
in vitro maturation medium increased oocyte maturation rate, and subsequent fertilization and blastocyst development rates [
17,
18]. However, the addition of AST has not been studied in IVF media. Therefore, this study focused on the effect of AST supplementation in IVF media by studying both the gamete fertilization medium and the embryo culture medium.
This study demonstrated that AST supplementation in the fertilization medium had a positive effect on the fertilization rate of mouse oocytes. However, the addition of AST into the culture medium had a detrimental effect on embryo development, resulting in decreased blastocyst formation rates. Therefore, considering the entire IVF process, supplementation with AST in the fertilization medium alone gave the best results for blastocyst development. Moreover, the resulting blastocysts were of higher quality, as measured by the proportion of hatching and hatched blastocysts and the blastocyst cell numbers. Routine supplementation of AST in all mediums of the IVF process should be avoided. Our study showed that the positive effect of an increased fertilization rate was diminished by poor embryo development rates, resulting in the same blastocyst rate as the control group (no AST supplementation in all steps), but with an even lower proportion of hatching and hatched blastocysts. Therefore, the routine addition of AST in all steps of the IVF process can have negative consequences.
There is a higher likelihood of oxidative stress occurring
in vitro than
in vivo. However, it remains unclear the extent to which the IVF process itself contributes to increased ROS levels. Moreover, the different steps of embryo production involve different levels of ROS. Therefore, the need for antioxidants varies depending on the stage of embryo production. Gamete fertilization in conventional IVF process appears to produce the highest levels of supraphysiologic ROS. The exact conditions under which natural fertilization takes place cannot be recreated in the laboratory setting. During natural fertilization, approximately 200 spermatozoa reach the egg in the Fallopian tube, but during IVF the number of spermatozoa inseminated into the drop of egg-containing medium is many times higher. ROS is generated during the normal aerobic metabolism of spermatozoa and oocytes, immature spermatozoa, dead spermatozoa, and leukocytes. This natural level of ROS is less than the amount generated by the large number of inseminated spermatozoa in conjunction with the lack of the reproductive systemŌĆÖs natural antioxidant defense mechanism. Excess ROS results in oocyte damage, sperm destruction, and loss of the ability to fertilize the oocyte. Sperm plasma membranes are composed of polyunsaturated fatty acids, which are particularly vulnerable to the lipid peroxidation process. Moreover, spermatozoa lack cytoplasmic enzymes and antioxidant mechanisms, making them susceptible to oxidative damage and allowing them to act as ROS carriers [
19]. In practice, it is important to minimize ROS damage by using a lower concentration of inseminated sperm and minimizing the period of sperm-oocyte co-incubation. The addition of antioxidants to reduce excess ROS and minimize oxidative damage is a viable option. Our study showed a significant increase in the fertilization rate of mouse oocytes after supplementing the fertilization medium with AST. Moreover, reducing oxidative damage to gametes led to the formation of a higher number of blastocysts and high-quality embryos.
In contrast, our study showed that AST supplementation in the culture medium impaired embryo development, with a significant decrease in the blastocyst formation rate when compared to the control group. The negative result may be because the embryo culture conditions did not produce ROS at supraphysiologic levels, or it could be from an excessive supplemental dose of AST. A low level of ROS is important for normal embryo development [
20]. Excess antioxidant supplementation can exert pro-oxidant activity, thereby inducing oxidative stress deleterious to the gamete/embryo [
21]. Therefore, the use of antioxidants should be limited to conditions that have a known likelihood of oxidative stress. Moreover, antioxidant potency and concentrations need to be carefully defined so that only the optimal amount is used to counteract the excess ROS, while maintaining a physiologic level of ROS.
Maintaining pro-oxidant/antioxidant equilibrium with antioxidant supplementation is difficult because of differences in ROS levels at each stage of development and because there is no standardized value for ROS. Moreover, the optimal concentration of antioxidants is important because antioxidants can have negative as well as positive effects, depending on the concentration of the supplement in the medium. Therefore, before supplementing the in vitro medium with an antioxidant, one should ask whether the supplement is indicated, and if the answer is ŌĆ£yes,ŌĆØ what concentration should be used. These issues remain subjects for further research.
In conclusion, AST supplementation in the fertilization medium alone resulted in the highest blastocyst formation rate and high-quality blastocysts. However, AST supplementation in the embryo culture medium was found to impair embryo development.