 |
 |
- Search
| Clin Exp Reprod Med > Volume 49(2); 2022 > Article |
|
Abstract
Objective
Y chromosome microdeletions are the second most common genetic cause of male infertility after Klinefelter syndrome. The aim of this study was to determine the patterns of Y chromosome microdeletions among infertile Mongolian men.
Methods
A descriptive study was performed on 75 infertile men from February 2017 to December 2018. Y chromosome microdeletions were identified by polymerase chain reaction. Semen parameters, hormonal levels, and testis biopsy samples were examined.
Infertility occurs in 10%–15% of all couples worldwide, and male infertility is responsible for 40%–50% of infertility cases [1,2]. The prevalence of infertility in Mongolia was 8.7% in 2003 and 11.6% in 2013 [3]. According to findings from the Child and Maternity Hospital, male factor infertility constitutes 25.6% of all infertility cases [4]. Y chromosome microdeletions are the second most common genetic cause of male infertility. In the general population, Y chromosome microdeletions occur in 1 in 4,000 men, but the frequency is significantly higher among infertile men. The association between Y chromosome microdeletions and defective spermatogenesis has been previously studied. The incidence of Y chromosome microdeletions is 2%–10% or even higher among azoospermic patients with no sperm count, or oligospermia patients with a sperm count of less than 5 million/mL [5]. The distal end of the long arm of the Y chromosome includes the azoospermia factor (AZF) locus, which contains the genes necessary for spermatogenesis. The AZF locus has been mapped to a region in band q11.23 of the Y chromosome. Microdeletions occur in the AZF region on the long arm of the Y chromosome, which includes AZFa, AZFb, and AZFc [6]. The diagnosis of Y chromosome microdeletions can establish the cause of the patient’s azoospermia and oligozoospermia and enable a prognosis to be formulated.
The purpose of this study was to investigate the frequency of Y chromosome microdeletions among infertile men who visited the Mon-CL Fertility Center, Ulaanbaatar, Mongolia, for evaluation, and to introduce a modern infertility diagnosis, contributing to further treatment. The standard method applied by the European Academy of Andrology/European Molecular Genetics Quality Network (EAA/EMQN) was used to evaluate patients. The data from this study demonstrate a low frequency (2.66%) of Y chromosome microdeletions in azoospermic and severe oligozoospermic infertile men in the Mongolian population.
Having obtained approval from the local institutional review board, this prospective descriptive study was carried out from February 2017 to December 2018. The inclusion criterion was as follows: screening for microdeletions was carried out only in men affected by azoospermia or severe oligozoospermia (sperm concentration of less than 5 million/mL). Patients were excluded if they had (1) semen analysis only performed once; (2) treatment with chemotherapeutic agents, radiotherapy, or testicular tumors; (3) karyotype abnormalities; or (4) retrograde ejaculation. According to the National Statistics Center of Mongolia, there were approximately 853,018 men (50.6% of the total male population) from the age of 15 to 49 in 2019. The confidence interval for the results using the given formula is 95%, with percentiles ranging from 10th to 95th in this study. Seventy-five azoospermic and severe oligozoospermic infertile patients were included in this study.
In all participants, semen analysis was performed at least twice, at 1-month intervals, following 3–7 days of sexual abstinence. These samples were collected and examined after 30 minutes of liquefaction. The mean values from different semen analyses were reported and the average results were used. The reference values set by the World Health Organization in 2010 were used: a sperm count over 15 million/mL was considered normal, a sperm count of ≤5 million/mL was defined as severe oligozoospermia, and the absence of sperm was defined as azoospermia.
Blood was taken from all participants for DNA analysis. DNA was extracted using DNA extraction kits (Chorosh Onosh, Ulaanbaatar, Mongolia). Next, DNA amplification by multiplex polymerase chain reaction (PCR) was performed using sequence-tagged sites primers for the AZFa sub-region (sY84 and sY86), the AZFb sub-region (sY127 and sY134), the AZFc sub-region (sY254, sY255), and the sex-determining region of the Y gene (sY14). Samples showing microdeletions on the first screening were verified by subsequent multiplex PCR amplification two more times. A complete description of the primers used for detecting Y-chromosome microdeletions and the amplification sets is shown in Table 1. The multiplex PCR amplification conditions were optimized as follows: initial denaturation at 95°C for 10 minutes, followed by 32 cycles at 95°C for 30 seconds, 60°C for 90 seconds, and 72°C for 60 seconds; with a final extension at 72°C for 1 minute. The PCR products were separated by electrophoresis on a 1.6% agarose gel stained with ethidium bromide. They were then viewed under ultraviolet trans-illumination. Negative controls with a DNA template were included with each reaction.
Serum samples were obtained by venipuncture from all participants to measure levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and total testosterone (TT). The serum samples were allowed to clot for 30 minutes and the samples were separated by centrifugation for 10 minutes. All hormone assays were estimated using the Elecsys FSH, Elecsys LH, and Elecsys TT on a Cobas e 411 analyzer (Roche Diagnostics, Indianapolis, IN, USA).
Multiple testicular sperm extraction (TESE) procedures were performed in azoospermic patients for diagnosis and treatment. If sperm was not retrieved by TESE, these testicular samples were fixed in 10% formalin solution, processed, embedded with paraffin, sectioned, stained with hematoxylin and eosin, and then examined for histological anomalies. Based on the most predominant and favorable histopathological pattern, testicular histology was classified as normal spermatogenesis, hypospermatogenesis, maturation arrest, Sertoli cell-only syndrome, or tubular hyalinization.
All collected data were evaluated with the Statistical Package for GraphPad Prism ver. 9.0.0 (GraphPad, San Diego, CA, USA). The data were presented as median and range, or number and percentage. The unpaired t-test was used for comparisons, and p-values of <0.05 were considered to indicate statistical significance.
This was a descriptive study conducted at the Mon-CL Fertility Center, Ulaanbaatar, Mongolia. The study was approved by the Institutional Review Board of the Mongolian National University of Medical Sciences (IRB No. 2017/3-05). Each patient who agreed to participate in the study provided written informed consent before enrollment.
Patient characteristics are presented in Figure 1. The patients’ age ranged from 24 to 46 years (34.51±5.42 on average). The average infertility period was 7.4±5.1 years. The mean body weight was 87.92±14.41 kg, and the average body mass index was 27.85±5.25 kg/m2. A total of 1,007 men underwent semen analyses from February 2017 to December 2018. From the total sample, 75 patients who were diagnosed with infertility were analyzed for Y chromosome microdeletions. Six patients (8.0%) had severe oligozoospermia and patients (92.0%) patients had azoospermia. From this group, 39 patients underwent TESE. The sperm retrieval rate was 64.1% (25 of 39 patients). Testicular samples from the 14 patients whose sperm was not retrieved were sent to the histopathology laboratory for analysis.
The mean age of azoospermic patients was 34.6±5.89 years old, and severe oligozoospermic patients averaged 33.7 years old. The average pH of the samples from all participants was 7.7. The mean semen volume of all patients was 3.05 mL (range, 0.2-7 mL). The mean semen volume was 2.94±1.79 mL in patients with azoospermia and 3.96±1.62 mL in those with severe oligozoospermia. The average sperm count or concentration in 1 mL of semen in patients with severe oligozoospermia was 1.60±1.64 million/mL (Table. 2).
Seventy-five patients were analyzed for Y chromosome microdeletions. A microdeletion in the Y chromosome was detected in the AZFa region (sY84 and sY86) in 1 patient and partially detected in the AZFc region (sY254) in 1 other patient. Deletions in the AZFb region were not detected (Figure. 2). Thus, Y chromosome microdeletions were detected in 2 patients (2.66%) (Table 3). PCR results of the 75 patients are shown in Supplementary Figure 1. The age of the patient with a partial deletion of AZFc was 40 years; he was diagnosed with azoospermia, did not undergo TESE, his FSH level was 23.85 mIU/mL, his LH level was 13.01 mIU/mL, and his TT level was 4.06 ng/mL. The age of the patient with an AZFa microdeletion was 31 years; this patient had azoospermia, sperm was not retrieved from the TESE procedure, and the histologic examination results showed Sertoli cell-only syndrome. His FSH level was 58.0 mIU/mL, his LH level was 12.0 mIU/mL, and his TT level was 5.0 ng/mL (Table 4). The hormone levels of the Y chromosome microdeletion group and the non-deletion group were compared. The average FSH level of the two patients (2.66%) with a microdeletion was 40.93±17.07 mIU/mL; the average LH level was 12.5±0.71 mIU/mL, and the average TT level was 4.53±0.66 ng/mL. In the 73 patients (97.4%) with no microdeletions, the average FSH level was 14.86±14.58 mIU/mL, the average LH level was 8.17±5.41 mIU/mL, and the average TT level was 3.09±2.16 ng/mL (Table 5). The mean level of FSH in the Y-chromosome microdeletion group was significantly higher than in the non-deletion group (p=0.016). The LH and TT levels showed no significant differences.
Thirty-nine patients with azoospermia underwent a TESE procedure for intracytoplasmic sperm injection under regional anesthesia. In this surgical procedure, a median raphe incision was made in the scrotum, the tunica vaginalis was opened, and the testis was delivered through the incision. A large piece of testicular tissue was obtained from the incision. The specimens were examined by an embryologist and were analyzed for the presence of spermatozoa when all tubules were teased apart. If no spermatozoa were found, the specimen was taken for histopathological examination. Of the 75 patients, 39 patients received TESE, 25 (64.1%) had sperm retrieved, and 14 (35.9%) had no sperm retrieved. The average age of the sperm retrieval group was 36.7±5.39 years, and the average age of the unsuccessful group was 31.8±3.37 years. The FSH level of patients undergoing TESE was 6.31±4.67 mIU/mL in the sperm retrieval group and 21.87±15.08 mIU/mL in the sperm non-retrieval group (p<0.001). The LH hormone level of patients undergoing TESE was 5.97±2.8 mIU/mL in the sperm retrieval group and 10.35±5.94 mIU/mL in the sperm non-retrieval group (p=0.016). Comparisons of variables including age and serum hormone levels according to sperm recovery from TESE were conducted. Serum FSH and LH levels were significantly lower in the sperm retrieval group. Patients were significantly younger in the sperm non-retrieval group. The other variables were found to be comparable between the two groups. Histopathological examinations showed Sertoli cell-only syndrome in 12 patients (85.71%), and seminiferous tubule hyalinization in two patients (14.29%) (Figure 3).
Y chromosome microdeletions are one of the most common causes of male infertility [5] . The Y chromosome AZF region contains many genes that are important for spermatogenesis. The region known as the AZF includes AZFa, AZFb, and AZFc [7]. The AZFa region contains USP9Y and DBY. The AZFb region contains CDY2, EIF1AY, HSFY, PRY, RBMYL1, RPS4YS, SMCY, and XKRY. The AZFc region contains BPY2, CDY1, CSPG4LY, DAZ, and GOLGA2LY [8]. A study by Tiepolo and Zuffardi [9] demonstrated that Y chromosome microdeletions are involved in testicle differentiation and testicle maturation. Y chromosome microdeletions play an important role in predicting sperm extraction from testes. Some studies have shown that Y chromosome microdeletions are associated with testicular cancer and recurrent pregnancy loss [10,11].
Our Y chromosome microdeletion study is the first-ever study in Mongolia carried out to investigate this issue among infertile patients. Y chromosome microdeletions were found in two (2.66%) of 75 patients with azoospermia and severe oligozoospermia. The reported incidence of Y chromosome microdeletions in infertile men varies between studies from 1% to 55% [12,13].
According to a 2008 report, the frequency of AZF microdeletions among infertile men was less than 2.5% in Sweden, Germany, and Austria, whereas it was 10% (the highest) in Australia, China, and Brazil [5]. A comparative study carried out across Asia among patients with idiopathic azoospermia or severe oligozoospermia showed frequencies of 19.4% in China, 10.6%–11.7% in Taiwan, 15.8% in Japan, 9.6%–12.0% in India, 3.2% in Saudi Arabia, 3.3% in Turkey, and 2.6% in Kuwait [14-16]. We used six different markers—for the AZFa region, sY84 and sY86; for the AZFb region, sY127 and sY134; and for the AZFc region, sY254 and sY255—according to the guidelines published by the EAA/EMQN. In a 2012 study of Y chromosome microdeletions in 115 patients in Iran, 1.7% showed deletions in the AZFc and AZFbc regions. Those researchers used the same six markers that we used in our study [17]. In a 2011 study, Akin et al. reported Y chromosome microdeletions in 7 patients (3.93%) among 178 infertile men. They were detected in the AZFc and AZFa regions [18]. In a study of 1,738 infertile men by Zhang et al. [19] in China, the frequency of Y chromosome microdeletions was 8.57%. Of note, the frequency of microdeletions in AZFa was 2.2%. In a study of 3,654 men by Totonchi et al. [20], the frequency of Y chromosome deletions was 5.06%. The proportion of deletions in AZFa was 2.16%, which is similar to our results [19]. Most patients with AZFa deletions were diagnosed with Sertoli cell-only syndrome [21]. In a case with the complete deletion of AZFa, no sperm were retrieved. However, in cases of partial deletion, it has been reported that sperm can be retrieved by TESE [21,22]. In our study, the patient with a Y chromosome AZFa microdeletion was 31 years old, with azoospermia; no sperm was retrieved from his testis, and a histologic examination showed Sertoli cell-only syndrome. These results are similar to those reported by Kamp et al. [21].
Both patients with Y chromosome microdeletions had azoospermia, and FSH and LH hormone levels were higher than normal. The FSH level was 40.93±17.07 mIU/mL and the LH level was 12.5±0.71 mIU/mL, but there was a significant difference in the FSH hormone level compared to the non-Y chromosome microdeletion group. These results are similar to the results reported by Wang et al. [23] and Kumar et al. [24].
The mean age of patients in the sperm retrieval group was 38.3±5.1 years, while the mean age of the sperm non-retrieval group was 31.5±3.63 years. This difference was statistically significant. A study by Tsai et al. [25] showed that in vitro fertilization (IVF) results, pregnancy rates, and miscarriage rates were correlated with male age. According to the results of a micro-TESE study by Enatsu et al. [26], the average age of the patients in whom sperm were retrieved from the testis was 35.0±5.6 years, while the average age of the sperm non-retrieval group was 33.2±4.9 years (p<0.05).
The 14 patients with no sperm retrieved by TESE were diagnosed with testicular histopathology. Histopathological examination showed Sertoli-cell-only syndrome in 12 patients (85.71%) and seminiferous tubule hyalinization in 2 patients (14.29%). Of the 25 patients who had sperm retrieved from TESE, 18 patients (72%) had IVF treatment. Two of these patients (11.1%) conducted embryo banking, and 16 (88.9%) had an embryo transfer. The partners of six patients (37.5%) had successful clinical pregnancies. One partner gave birth to twins, three delivered single babies, and two patients had missed abortions during the first trimester. The current data show that there is a low frequency of Y chromosome microdeletions in azoospermic and severe oligozoospermic infertile men in the Mongolian population. In patients with non-obstructive azoospermia, AZFa, AZFb, and AZFb/c microdeletions occur in 1%–2% of cases, but sperm is not retrieved by TESE, so there is no need for unnecessary TESE procedures. In patients with AZFc microdeletions, sperm formation functions normally. IVF can be performed with the sperm of a patient with microdeletions in the AZFc region, potentially resulting in a successful pregnancy. However, microdeletions within the AZFc region will likely be inherited by male children. We recommend further studies with a larger group of patients and control subjects screened for this microdeletion in order to confirm our results.
Supplementary material
Supplementary material can be found via https://doi.org/10.5653/cerm.2021.05099.
Supplementary Figure 1.
Polymerase chain reaction (PCR) results of patient 1 (P1) to patient 75 (P75). Lane 1: size marker; lane 2: negative control (D.W); lane 3: negative control (female patient blood); lane 4: positive control (sY127); lane 5: positive control (sY14); lane 6: AZFa- sY84; lane 7: AZFa-sY86; lane 8: AZFb-sY127; lane 9: AZFb-sY134; lane 10: AZFc-sY254; lane 11: AZFc-sY255.
Acknowledgments
The authors would like to thank Dr. Sang-Jin Song for providing a Y chromosome microdeletion setting for this research. We would also like to thank Dr. Enkhee. O, the Head of the Department of Adult Pathology, National Center for Pathology of Mongolia for analyzing the biopsy results and Dr. Gantumur Battogtokh, R&D Center, Upex-Med Co. Ltd, South Korea, who helped with the writing and submission of the manuscript. The authors would also like to express their gratitude to all individuals who participated as patients in this study.
Figure 1.
Flowchart of study participants. A total of 1,007 men underwent semen analyses. Of these, 75 patients who were diagnosed with infertility were analyzed for Y chromosomal microdeletions. Six patients (8.0%) had severe oligozoospermia and 69 patients (92.0%) had azoospermia. Thirty-nine underwent testicular sperm extraction (TESE). Sperm was retrieved from 25 patients (64.1%), but not from 14 patients (35.9%). The tissue samples of these 14 patients were sent for biopsies. PCR, polymerase chain reaction.
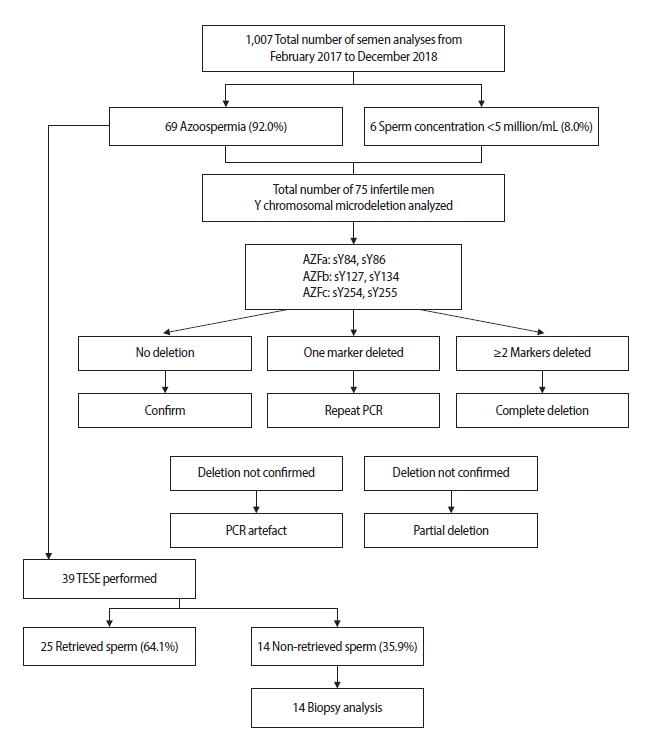
Figure 2.
Polymerase chain reaction (PCR) results of Y chromosome microdeletions. (A) A representative picture of the Y chromosome non-deletion group. Lane 1: size marker; lane 2: negative control; lane 3: negative control (female patient blood); lane 4: positive control (sY127); lane 5: positive control (sY14); lane 6: AZFa- sY84; lane 7: AZFa-sY86; lane 8: AZFb-sY127; lane 9: AZFb-sY134; lane 10: AZFc-sY254; lane 11: AZFc-sY255. (B) A representative picture of an AZFc (sY-254) microdeletion. Lane 1: size marker; lane 2: negative control (distilled water); lane 3: negative control (female patient blood); lane 4: positive control (sY127); lane 5: positive control (sY14); lane 6: AZFa- sY84; lane 7: AZFa-sY86; lane 8: AZFb-sY127; lane 9: AZFb-sY134; lane 10: AZFc-sY254; lane 11: AZFc-sY255. (C) A representative picture of an AZFa (sY84 and sY86) microdeletion. Lane 1: size marker; lane 2: negative control (distilled water); lane 3: negative control (female patient blood); lane 4: positive control (sY127); lane 5: positive control (sY14); lane 6: AZFa- sY84; lane 7: AZFa-sY86; lane 8: AZFb-sY127; lane 9: AZFb-sY134; lane 10: AZFc-sY254; lane 11: AZFc-sY255. SM, size marker; NC, negative control; PC, positive control.

Figure 3.
Patterns of Sertoli cell-only syndrome. (A) Testicular atrophic tubules with hyalinization (H&E, 4×10, 10×40). (B, C) Tubules with thickened basal membranes are lined by Sertoli cells, some of which are altered in shape and detached from the basal membrane, and devoid of germ cells (H&E, 4×10, 10×40). (D) Expansion of interstitial space along with increased connective tissue. Atrophic appearance in the seminiferous tubules. Basement membrane thickening (H&E, 4×10, 10×10).
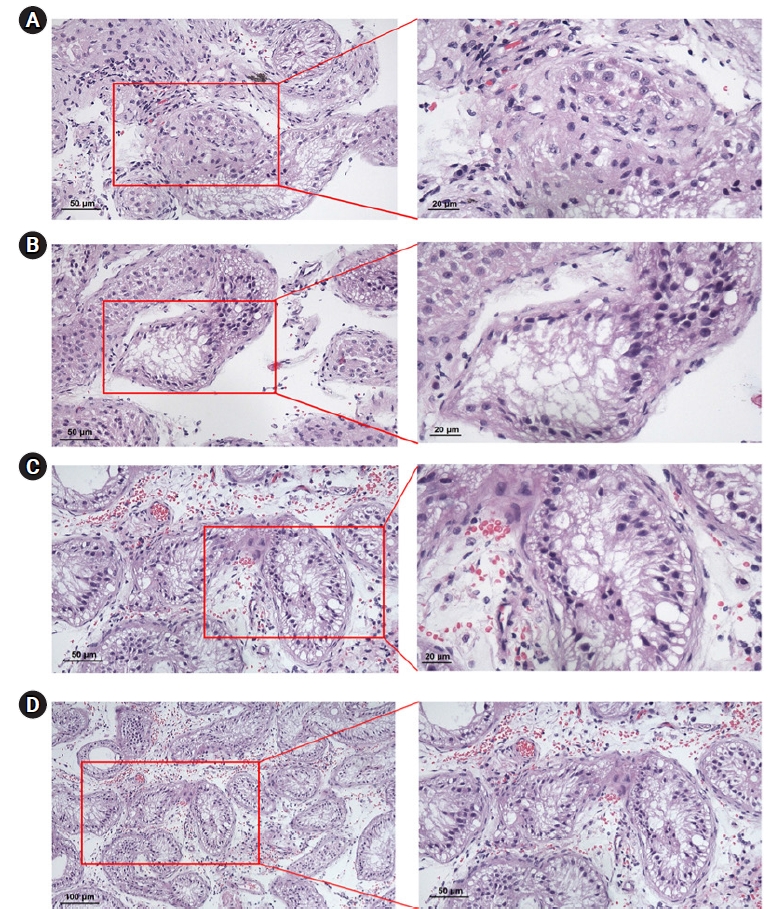
Table 1.
The STS primer set used to detect Y chromosome microdeletions
Table 2.
Results of semen analysis
Table 3.
Prevalence of Y chromosome microdeletions
| Deleted loci | Case | Prevalence (%) |
|---|---|---|
| AZFa | 1 | 1.33 |
| AZFb | 0 | 0 |
| AZFc | 1 | 1.33 |
| Total | 2 | 2.66 |
Table 4.
Clinical features of the Y chromosome microdeletions
Table 5.
Hormone levels of the Y chromosome microdeletion and non-deletion groups
| Hormone level | Y chromosome microdeletion group (n=2) | Y chromosome non-deletion group (n=73) | p-value |
|---|---|---|---|
| FSH (mIU/mL) | 40.93±17.07 | 14.86±14.58 | 0.016a) |
| LH (mIU/mL) | 12.5±0.71 | 8.17±5.41 | 0.268 |
| TT (ng/mL) | 4.53±0.66 | 3.09±2.16 | 0.357 |
References
1. Gnoth C, Godehardt E, Frank-Herrmann P, Friol K, Tigges J, Freundl G. Definition and prevalence of subfertility and infertility. Hum Reprod 2005;20:1144-7.


2. De Kretser DM, Baker HW. Infertility in men: recent advances and continuing controversies. J Clin Endocrinol Metab 1999;84:3443-50.


3. Purevtogtokh M, Batmunkh G. Determining the spread of infertility based on the data of the social indicators. Child Maternity Health Stud 2017;1:180-2.
4. Bayasgalan G. The major clinical forms and risk factors of male infertility in Mongolia. Ulaanbaatar: Mongolian National University of Medical Sciences; 2005.
5. Krausz C, Hoefsloot L, Simoni M, Tuttelmann F; European Academy of Andrology; European Molecular Genetics Quality Network. EAA/EMQN best practice guidelines for molecular diagnosis of Y-chromosomal microdeletions: state-of-the-art 2013. Andrology 2014;2:5-19.


6. Repping S, Skaletsky H, Lange J, Silber S, Van Der Veen F, Oates RD, et al. Recombination between palindromes P5 and P1 on the human Y chromosome causes massive deletions and spermatogenic failure. Am J Hum Genet 2002;71:906-22.



7. Vogt PH, Edelmann A, Kirsch S, Henegariu O, Hirschmann P, Kiesewetter F, et al. Human Y chromosome azoospermia factors (AZF) mapped to different subregions in Yq11. Hum Mol Genet 1996;5:933-43.


8. Vogt PH. Genomic heterogeneity and instability of the AZF locus on the human Y chromosome. Mol Cell Endocrinol 2004;224:1-9.


9. Tiepolo L, Zuffardi O. Localization of factors controlling spermatogenesis in the nonfluorescent portion of the human Y chromosome long arm. Hum Genet 1976;34:119-24.


10. Bor P, Hindkjaer J, Kolvraa S, Rossen P, von der Maase H, Jorgensen TM, et al. Screening for Y microdeletions in men with testicular cancer and undescended testis. J Assist Reprod Genet 2006;23:41-5.



11. Dewan S, Puscheck EE, Coulam CB, Wilcox AJ, Jeyendran RS. Y-chromosome microdeletions and recurrent pregnancy loss. Fertil Steril 2006;85:441-5.


12. van der Ven K, Montag M, Peschka B, Leygraaf J, Schwanitz G, Haidl G, et al. Combined cytogenetic and Y chromosome microdeletion screening in males undergoing intracytoplasmic sperm injection. Mol Hum Reprod 1997;3:699-704.


13. Foresta C, Ferlin A, Garolla A, Moro E, Pistorello M, Barbaux S, et al. High frequency of well-defined Y-chromosome deletions in idiopathic Sertoli cell-only syndrome. Hum Reprod 1998;13:302-7.


14. Lin YM, Lin YH, Teng YN, Hsu CC, Shinn-Nan Lin J, Kuo PL. Gene-based screening for Y chromosome deletions in Taiwanese men presenting with spermatogenic failure. Fertil Steril 2002;77:897-903.


15. Athalye AS, Madon PF, Naik NJ, Naik DJ, Gavas SS, Dhumal SB, et al. A study of Y chromosome microdeletions in infertile Indian males. Int J Hum Genet 2004;4:179-85.

16. Kumtepe Y, Beyazyurek C, Cinar C, Ozbey I, Ozkan S, Cetinkaya K, et al. A genetic survey of 1935 Turkish men with severe male factor infertility. Reprod Biomed Online 2009;18:465-74.


17. Saliminejad K, Sadeghi MR, Kamali K, Amirjannati N, Soltanghoraee H, Khorram Khorshid HR. Discrepancy in the frequency of Y chromosome microdeletions among Iranian infertile men with azoospermia and severe oligozoospermia. Genet Test Mol Biomarkers 2012;16:931-4.


18. Akin H, Onay H, Turker E, Ozkinay F. Primary male infertility in Izmir/Turkey: a cytogenetic and molecular study of 187 infertile Turkish patients. J Assist Reprod Genet 2011;28:419-23.



19. Zhang YS, Dai RL, Wang RX, Zhang HG, Chen S, Liu RZ. Analysis of Y chromosome microdeletion in 1738 infertile men from northeastern China. Urology 2013;82:584-8.


20. Totonchi M, Mohseni Meybodi A, Borjian Boroujeni P, Sedighi Gilani M, Almadani N, Gourabi H. Clinical data for 185 infertile Iranian men with Y-chromosome microdeletion. J Assist Reprod Genet 2012;29:847-53.



21. Kamp C, Huellen K, Fernandes S, Sousa M, Schlegel PN, Mielnik A, et al. High deletion frequency of the complete AZFa sequence in men with Sertoli-cell-only syndrome. Mol Hum Reprod 2001;7:987-94.


22. Krausz C, Forti G. Clinical aspects of male infertility. Results Probl Cell Differ 2000;28:1-21.


23. Wang LQ, Huang HF, Jin F, Qian YL, Cheng Q. High frequency of Y chromosome microdeletions in idiopathic azoospermic men with high follicle-stimulating hormone levels. Fertil Steril 2005;83:1050-2.


24. Kumar R, Dada R, Gupta NP, Kucheria K. Serum FSH levels and testicular histology in infertile men with non obstructive azoospermia and Y chromosome microdeletions. Indian J Urol 2006;22:332-6.

- TOOLS
-
METRICS

-
- 1 Crossref
- Scopus
- 2,885 View
- 313 Download
- Related articles in Clin Exp Reprod Med
-
Effects of Y Chromosome Microdeletion on the Outcome of in vitro Fertilization.2007 March;34(1)






