|
 |
- Search
| Clin Exp Reprod Med > Volume 39(3); 2012 > Article |
Abstract
Objective
It is well known that fresh blastocyst transfer results in better pregnancy outcomes with a smaller number of transferred embryos compared with cleavage stage embryo transfer. However, in terms of frozen-thawed blastocyst transfer, only a few studies are available. We aimed to evaluate clinical outcomes of frozen-thawed embryo transfer (FET) with blastocysts.
Methods
Retrospective analysis of FET cycles with blastocysts (B-FET) between Jan 2007 and June 2009 was performed. Age-matched FET cycles with cleavage stage embryos (C-FET) during the same period were collected as controls. A total of 58 B-FET cycles were compared with 172 C-FET cycles and also compared with those of post-thaw extended culture blastocysts from frozen pronuclear stage embryos (22 cycles).
Recent advances in cell culture technique and sequential media are leading a shift in IVF practice from early cleavage stage embryo transfer (ET) to blastocyst transfer. Theoretically, blastocyst culture and transfer improves the synchronization of the endometrium and embryos and induces self-selection of viable embryos through the period of extended culture [1]. In practice, many investigators have reported that blastocyst transfers result in a higher implantation rate (IR) and better pregnancy outcomes with a smaller number of transferred embryos compared to cleavge stage ETs [1-3]. However, only a few studies have been performed to evaluate frozen-thawed embryo transfer (FET) with blastocysts.
The first successful pregnancy from a FET was reported in 1983 [4]. Cryopreservation prevents the wastage of supernumerary embryos and is now considered a vital process in a successful human IVF-ET cycle. The transfers of frozen-thawed embryos constitute about 20% of all ETs worldwide [5]. Several different freezing-thawing protocols have developed for each stage of embryos. These developments and improvements in cryopreservation method have helped to increase the clinical pregnancy rate (CPR) and IR. Though slow freezing is a classic method of cryopreservation for human reproductive cells, it requires expensive equipment and is a time-consuming process [6]. Hence, vitrification appears to be more attractive for the freezing of a small number of embryos at a time. Vitrification is less expensive as it does not use expensive instruments, and it is more time efficient, requiring several minutes as compared with 1 to 2 hours for slow freezing. Moreover, several reports show improved results in terms of the survival rate and clinical pregnancy rates with the application of vitrification [7,8]. Since 2007, our institute also has used the vitrification method for cryopreservation of blastocysts and its survival rate after thawing is comparable to slow freezing.
Recently, several investigators have reported that fresh ET can be replaced by FET [9-11]. According to one randomized controlled trial, FET showed a higher ongoing pregnancy rate (OPR) compared with fresh ET; however, the authors did not perform subgroup analysis depending on the stage of the transferred embryos [9]. According to previous studies, we hypothesized better pregnancy outcomes in FET with blastocysts than FET with cleavage stage embryos and fresh blastocyst transfer cycles and aimed to evaluate the clinical outcomes of FET with blastocysts.
Retrospective analysis of FET cycles with blastocysts between Jan 2007 and June 2009 in Cheil General Hospital, Seoul, Korea was performed. Age-matched FET cycles with cleavage stage embryos of the same period were collected as a control group. The cycles with donated oocytes and preimplantation genetic diagnosis were excluded. A total of 58 frozen-thawed blastocyst transfer cycles (B-FET) were compared with 172 FET cycles with cleavage stage embryos (C-FET). B-FET were also compared with 143 fresh blastocysts transfer (fB-ET) cycles and 22 cycles with post-thaw extended culture (PTEC)-blastocysts, which were cultured further to the blastocyst after thawing frozen pro-nucleus staged embryos (PN). 430 age-matched cycles with fresh cleavage stage embryos (fC-ET) of the same period were reviewed for additional comparison.
The ovarian stimulation was performed as usual as has been extensively described. Briefly, the patients underwent pituitary down-regulation with GnRH agonist or antagonist. When two or more follicles reached 18 mm in diameter, 10,000 units of hCG was administered and transvaginal ultrasound-guided oocyte pick-up (OPU) was performed 34 to 36 hours later. According to the quality of the sperm, insemination (conventional IVF) or microinjection (ICSI) was carried out 4 to 6 hours after OPU. After 16 to 18 hours, fertilization of the oocytes was checked and if more than 10 zygotes were acquired, cryopreservation was attempted with some PN. Then, fresh ET was performed on day 3 (cleavage stage ET) or day 5 (blastocyst transfer) and the rest of the blastocysts of a good grade were frozen.
PNs were frozen and thawed as previously reported [12]. Briefly, PNs were frozen using the slow freezing method with 1.5 M proplylene glycol and 0.1 M sucrose. The embryos were loaded into a 0.25 mL sterile straw (Bicef, L'Aigle, France) and then the straw was loaded into a programmable/controlled-rate freezing machine (Cryo-magic, Miraebiotech, Seoul, Korea). Frozen PNs were thawed by the rapid thawing method.
Blastocysts were vitrified with a pull and cut straw, which was constructed by pulling and cutting a 0.25 mL plastic sterile straw (Bicef). The blastocyst(s) were equilibrated in 7.5% ethylene glycol (EG) and 7.5% dimethyl sulfoxide (DMSO) for 20 to 25 minutes, and exposed in 15% EG and 15% DMSO in phosphate buffered saline (PBS) adding 0.5 M sucrose within 1 minute. Then, they were immediately plunged into the liquid nitrogen. For thawing, they were warmed in 1 M sucrose for 1 minute and 0.5 M sucrose for 3 minutes, and then washed in PBS.
The embryos with a normal morphology after thawing or PTEC were transferred to endometrium prepared with oral estrogen (6-8 mg oral daily, Progynova, Bayer Schering Pharma AG, Berlin, Germany) and progesterone (50 mg/mL intramuscular daily, Progesterone Injection USP in sesame oil, Watson Laboratories, Inc., Corona, CA, USA) as usual protocols of FET. Progesterone was administered until the 8th week of gestation for luteal support.
Clinical pregnancy and ongoing pregnancy were defined as the presence of a gestational sac on transvaginal ultrasound at the 5th to 7th weeks of gestation and the existence of a fetal heartbeat at approximately 12 weeks gestation. We compared patients' characteristics and the pregnancy outcomes of each group.
Statistical analysis was performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). Continuous characteristic values of each group, such as age and body mass index (BMI), were compared using the Student's t-test. Ordinary values such as the pregnancy rate were analyzed by the Žć2-test. A p-value<0.05 was reported as statistically significant.
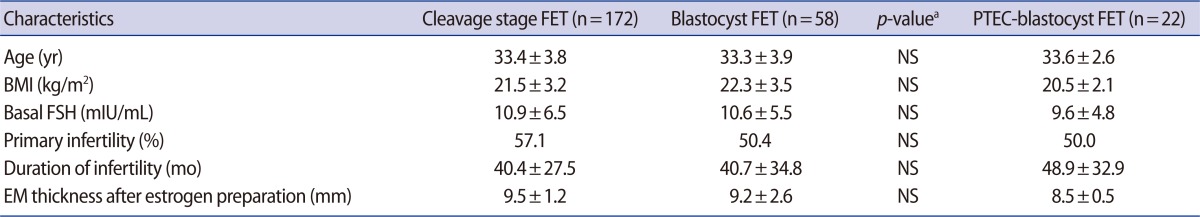
Age and BMI of the B-FET group were 33.3┬▒3.9 year and 22.3┬▒3.5 kg/m2, respectively. There was no difference in age, BMI, or basal FSH between the B-FET group and C-FET group (Table 1). The survival rates of embryos after thawing were similar (90.8% vs. 93.3%) between the two groups. No statistically significant difference was found in the IR (21.6% vs. 19.5%), CPR (43.1% vs. 44.2%), or OPR (39.7% vs. 40.7%) between the two groups. In B-FET, the mean number of transferred embryos was significantly lower (2.0┬▒0.7 vs. 3.1┬▒0.8, p<0.001) than that in C-FET and the multiple pregnancy rate (MPR, 15.0% vs. 26.3%) tended to be lower in B-FET, but it did not reach statistical significance (Table 2).
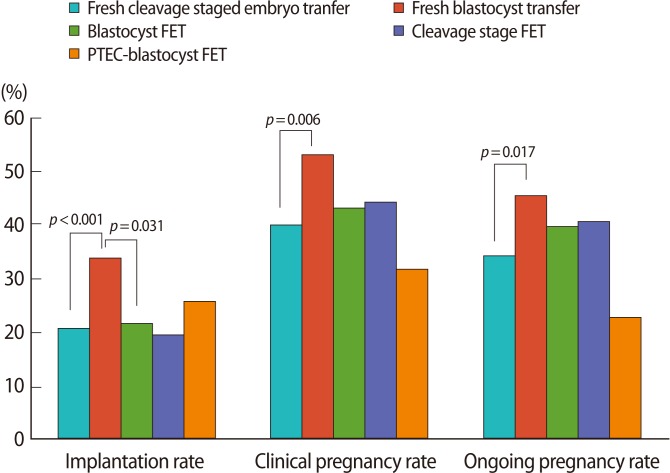
In additional analysis, in blastocyst transfers after PTEC (PTEC-blastocyst FET group), the number of ET (2.6┬▒0.8 vs. 2.0┬▒0.7, p=0.003) and MPR (15.0% vs. 62.5%, p=0.022) was shown to be significantly higher than in the B-FET group. However, there was no difference in other pregnancy outcomes (i.e., IR, CPR, or OPR) between the two groups (Table 2, Figure 1).
On the other hand, there was a significantly higher IR (33.8% vs. 20.7%, p<0.001), CPR (53.1% vs. 40.0%, p=0.006), and OPR (45.5% vs. 34.2%, p=0.017) in the fB-ET group compared with the fC-ET without any difference in patient characteristics or MPR (Figure 1).
Blastocyst FET may be better than fresh blastocyst transfer because it may undergo self-selection processes twice. The first selection is the avoidance of arrest through the extended culture and the second is the survival during the freezing-thawing processes. In practice, only a few studies about the superiority of blastocyst FET have been reported and each of them has presented different results. Some investigators have shown favorable pregnancy outcomes in blastocyst FET compared with other staged embryo FET [10,13]. Another study contradicted the former studies [14]. On the other hand, one study that compared blastocyst FET with fresh blastocyst transfer reported that blastocyst FET showed a lower IR but similar live birth rate compared to fresh blastocyst transfer with same graded embryos on the embryo grading system [15].
This study showed a higher IR and pregnancy rate in fresh blastocyst transfer than other fresh ET cycles as previous studies have reported (Figure 1). However, in FET, the blastocyst transfers did not show any benefit in pregnancy outcomes compared with cleavage stage FET. Moreover, these results were found not only in frozen-thawed blastocyst transfer but also in PTEC-blastocyst transfer. In addition, PTEC-blastocyst FET showed an extremely high MPR (62.5%). Nevertheless, we cannot exclude possible faults of a post-thawing extended culture system or developmental defects of blastocysts during the freezing-thawing process, and thus the results would be disappointing.
In many studies, vitrification has shown a better survival rate and similar or even higher pregnancy rate compared with the slow-freezing method [7,8]. It takes a short time to perform but needs a great deal of care for one embryo. Therefore, many IVF laboratories prefer the vitrification method for blastocyst freezing, which is performed with a small number of embryos at a time. For cryopreservation of PNs, slow-freezing is preferred. In this study, we also used vitrification for blastocysts and slow-freezing for PNs. This different freezing method may affect the study results.
Though we collected a relatively large number of control group (cleavage stage FET) cases, there are limitations from the small number of study group (blastocyst FET) cases. Indeed, the difference of about 10% in the MPR (26.3% in C-FET vs. 15.0% in B-FET) between the two groups did not reach statistical significance because of the small number of study population. Although the difference between IR and MPR in each group did not reach statistical significance, blastocyst FET tends to have a lower MPR. In addition, the number of transferred embryos was significantly lower in B-FET than in the other groups (Table 2).
In summary, blastocyst FET may not present better pregnancy outcomes, except the MPR, as fresh blastocyst transfer compared with other stage embryo FET. Therefore, this study suggests that it will be necessary to attempt to improve the post-thawing extended culture technique and conditions and to discover how to avoid possible defects after or during the freezing-thawing process of blastocysts prior to making the major effort to transfer blastocysts in FET cycles. A further large-scale randomized controlled study is needed.
References
1. Papanikolaou EG, Kolibianakis EM, Tournaye H, Venetis CA, Fatemi H, Tarlatzis B, et al. Live birth rates after transfer of equal number of blastocysts or cleavage-stage embryos in IVF. A systematic review and meta-analysis. Hum Reprod 2008;23:91-99.PMID: 17965420.


2. Papanikolaou EG, Camus M, Kolibianakis EM, Van Landuyt L, Van Steirteghem A, Devroey P. In vitro fertilization with single blastocyst-stage versus single cleavage-stage embryos. N Engl J Med 2006;354:1139-1146.PMID: 16540614.


3. Blake DA, Farquhar CM, Johnson N, Proctor M. Cleavage stage versus blastocyst stage embryo transfer in assisted conception. Cochrane Database Syst Rev 2007;(4): CD002118PMID: 17943767.


4. Trounson A, Mohr L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature 1983;305:707-709.PMID: 6633637.


5. Liebermann J, Dietl J, Vanderzwalmen P, Tucker MJ. Recent developments in human oocyte, embryo and blastocyst vitrification: where are we now? Reprod Biomed Online 2003;7:623-633.PMID: 14748959.


6. Loutradi KE, Kolibianakis EM, Venetis CA, Papanikolaou EG, Pados G, Bontis I, et al. Cryopreservation of human embryos by vitrification or slow freezing: a systematic review and meta-analysis. Fertil Steril 2008;90:186-193.PMID: 17980870.


7. Kolibianakis EM, Venetis CA, Tarlatzis BC. Cryopreservation of human embryos by vitrification or slow freezing: which one is better? Curr Opin Obstet Gynecol 2009;21:270-274.PMID: 19276976.


8. Stehlik E, Stehlik J, Katayama KP, Kuwayama M, Jambor V, Brohammer R, et al. Vitrification demonstrates significant improvement versus slow freezing of human blastocysts. Reprod Biomed Online 2005;11:53-57.PMID: 16102287.


9. Aflatoonian A, Oskouian H, Ahmadi S, Oskouian L. Can fresh embryo transfers be replaced by cryopreserved-thawed embryo transfers in assisted reproductive cycles? A randomized controlled trial. J Assist Reprod Genet 2010;27:357-363.PMID: 20373015.



10. Anderson AR, Weikert ML, Crain JL. Determining the most optimal stage for embryo cryopreservation. Reprod Biomed Online 2004;8:207-211.PMID: 14989799.


11. Li HZ, Qiao J, Chi HB, Liu P, Wu BS, Chen XN, et al. Comparison of multiple pregnancy rate following transfer of frozen-thawed or fresh embryos and analysis of related factors. Zhonghua Yi Xue Za Zhi 2009;89:2626-2628.PMID: 20137680.

12. Shin MR, Choi HW, Kim MK, Lee SH, Lee HS, Lim CK. In vitro development and gene expression of frozen-thawed 8-cell stage mouse embryos following slow freezing or vitrification. Clin Exp Reprod Med 2011;38:203-209.PMID: 22384443.



13. Kattera S, Shrivastav P, Craft I. Comparison of pregnancy outcome of pronuclear- and multicellular-stage frozen-thawed embryo transfers. J Assist Reprod Genet 1999;16:358-362.PMID: 10459518.



14. Moragianni VA, Cohen JD, Smith SE, Schinfeld JS, Somkuti SG, Lee A, et al. Outcomes of day-1, day-3, and blastocyst cryopreserved embryo transfers. Fertil Steril 2010;93:1353-1355.PMID: 19815194.


15. Shebl O, Ebner T, Sommergruber M, Sir A, Tews G. Cryopreserved blastocysts have a lower implantation but an equal live birth rate as compared to fresh blastocysts of the same quality - a case-control study. Acta Obstet Gynecol Scand 2009;88:944-947.PMID: 19562560.


Figure┬Ā1
Pregnancy outcomes of each frozen-thawed embryo transfer cycle compared with fresh cycles. FET, frozen-thawed embryo transfer; PTEC, post-thaw extended culture.
Table┬Ā2
Pregnancy outcomes of FETs
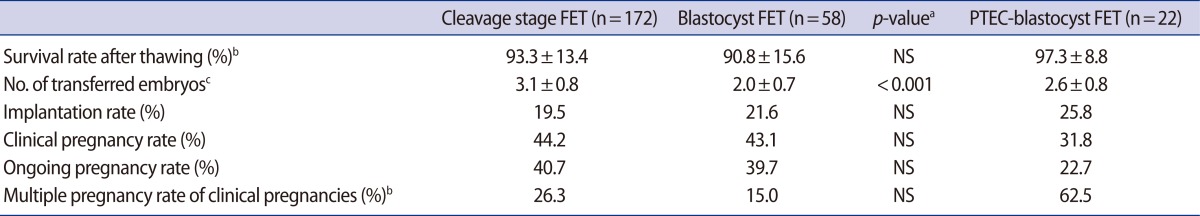
Values are presented as mean┬▒standard deviation.
FET, frozen-thawed embryo transfer; PTEC, post-thaw extended culture; NS, not significant.
ap-value presents the significance of the difference between cleavage staged FET and blastocyst FET; bSignificantly different between B-FET and PTEC-blastocyst FET (p<0.05); cSignificantly different between C-FET and B-FET.