|
 |
- Search
| Clin Exp Reprod Med > Volume 38(2); 2011 > Article |
Abstract
Objective
To investigate the effects of pioglitazone on controlled ovarian stimulation (COS), IVF outcomes, and follicular fluid (FF) cytokine concentrations in patients with polycystic ovary syndrome (PCOS).
Methods
Eighty-six infertile patients with PCOS resistant to clomiphene citrate were randomized to receive pioglitazone (30 mg/day) or placebo on the starting day of oral contraceptive (OC) pretreatment, followed by an IVF protocol using a GnRH antagonist. Pioglitazone or placebo was administered once daily from the starting day of OC to the day of hCG injection.
Results
Total dose and days of recombinant follicle-stimulating hormone administered, and the numbers of retrieved and mature oocytes, were significantly lower in the pioglitazone group than in the control group. FF tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) concentrations at oocyte retrieval were also significantly lower in the pioglitazone group. The clinical pregnancy rate was higher and the incidence of severe ovarian hyperstimulation syndrome was lower in the pioglitazone group, but the differences were not statistically significant.
Polycystic ovary syndrome (PCOS), the most common endocrine-gynecological disorder in women of reproductive age, is characterized by menstrual irregularity, infertility, obesity and hyperandrogenism [1,2]. Although the exact pathogenesis of these complex clinical presentations is not completely understood, insulin resistance is considered important [3]. Patients with PCOS are therefore treated with insulin sensitizing agents such as the biguanide metformin, and thiazolidinediones have been tested in these patients. Although troglitazone is the most studied thiazolidinedione, it was withdrawn from the market because of serious hepatotoxicity [2].
We previously investigated the effects of pioglitazone, a new thiazolidinedione derivative, on ovarian stimulation, IVF outcome, and intraovarian stromal blood flow in patients with PCOS. We found that pioglitazone therapy reduced intraovarian stromal blood flow and may improve ovarian stimulation response and IVF outcomes in PCOS patients [4].
Chronic low grade inflammation and imbalances between pro- and anti-inflammatory cytokines may also be involved in the pathogenesis of PCOS. Tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) are principal pro-inflammatory mediators, and have been shown to influence ovarian function, ovulation, fertilization and implantation in women with PCOS [5]. In addition, the concentrations of circulating inflammatory markers have been correlated with obesity and insulin resistance in patients with PCOS [6]. Furthermore, patients with PCOS were found to have higher serum and follicular fluid (FF) concentrations of TNF-α and IL-6 than women without PCOS [7]. We therefore investigated the effect of pioglitazone on the concentrations of TNF-α and IL-6 in ovarian FF in women with PCOS undergoing IVF-ET.
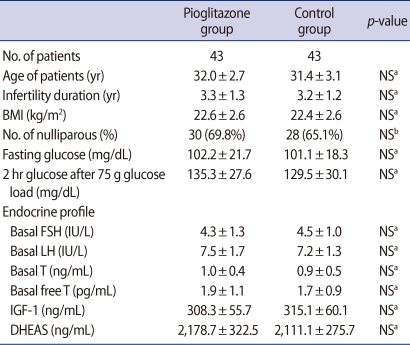
Eighty-six infertile women with PCOS were prospectively recruited to this randomized study, which was performed at a university-based infertility clinic at the Asan Medical Center, Seoul, South Korea. Patients were diagnosed with PCOS based on the revised diagnostic criteria of the 2003 Rotterdam consensus [8]. All had failed to ovulate or conceive after repeated treatment with up to 5 cycles of 150 mg/day clomiphene citrate (CC) on each of 5 days. They don't have any other etiologic factors of infertility. All patients were in good health with normal thyroid, hepatic, and renal functions, and all had experienced spontaneous onset of puberty and normal sexual development. Blood samples were collected from each patient on menstrual cycle day (MCD) 3 for basal measurements of glucose, sex hormones, insulin-like growth factor-1 (IGF-1) and DHEAS. Patient characteristics are shown in Table 1.
Sample size was determined in test of superiority. Patients were randomized to treatment with pioglitazone (n=43) or placebo (n=43) using sealed envelopes. Prior to ovarian stimulation, all patients were pretreated for 3 weeks with a monophasic oral contraceptive (OC, Diane35; Schering AG, Berlin, Germany). Patients were prescribed 30 mg pioglitazone (Actos; Takeda, Osaka, Japan) or placebo once daily from the starting day of OC to the day of hCG (Ovidrel; Serono, Geneva, Switzerland) injection.
All patients provided written informed consent, and the study protocol was approved by the institutional review board of the University of Ulsan College of Medicine, Asan Medical Center.
Controlled ovarian stimulation (COS) was induced with 50-150 IU of recombinant human follicle-stimulating hormone (rhFSH, Puregon; Organon, Oss, Netherland), starting on MCD 3. Initial dose of rhFSH was determined according to the patient's age and body mass index. The dose of rhFSH was adjusted every 3-4 days based on ovarian response, as determined by ultrasound scans. When the lead follicle reached a diameter of 13 to 14 mm, we started treatment with a GnRH antagonist (Cetrotide; ASTA Media, Frankfurt am Main, Germany), continuing up to the day of hCG injection. When one or more follicles reached a mean diameter of ≥18 mm, 250 µg hCG was administered subcutaneously to induce follicular maturation. Oocytes were retrieved 35 to 36 hours later, with one to four embryos transferred to the uterus three days later.
The luteal phase was supplemented with 90 mg of vaginal progesterone gel (Crinone 8%; Serono, SA, Geneva, Switzerland) once daily, starting on the day of oocyte retrieval. Pregnancies were determined by an increase in serum β-hCG concentration and transvaginal ultrasonographic evidence of a gestational sac. Serum concentrations of β-hCG were measured 11 days after embryo transfer (ET) by radioimmunoassay using an hCG MAIAclone kit (Serono Diagnostics, Woking, UK), with inter- and intraassay variances of <10% and 5%, respectively. A clinical pregnancy was defined as the presence of a gestational sac on ultrasonography.
Ovarian FFs were obtained from at least 2 follicles (range, 17-21 mm) at oocyte retrieval from each patient. Each follicle was aspirated separately, and fluids were collected in 15 mL conical tubes. A pooled FF sample from each patient was obtained by combining equal aliquots from two or more fluid collection tubes. Each fluid sample was centrifuged (2,000 g) to remove cell debris, and all supernatants were stored at -20℃.
The concentrations of IL-6 and TNF-α in each FF sample were measured by a solid-phase enzyme-linked immunosorbent assay (Medgenix Diagnostics, Fleurus, Belgium). Intraassay coefficients of variation were 3.3% for TNF-α and 4.2% for IL-6.
Results are reported as the mean±SD. Student's t-test was used to compare the mean values. The Chi-square test and Fisher's exact test were used to compare the fractions. Statistical significance was defined as p<0.05. The SPSS statistical package for Windows, ver. 11.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses.
Baseline patient demographic characteristics and baseline hormone concentrations were similar in the two groups (Table 1). There were no differences in fasting plasma glucose concentrations and two hour plasma glucose after 75 g glucose load. Ovarian stimulation characteristics are summarized in Table 2. The duration of stimulation (p<0.001) and the total gonadotropin dose (p=0.002) were significantly lower in the pioglitazone than in the control group, as were the numbers of follicles ≥14 mm (p=0.002) and 11 to 13 mm (p<0.001). The numbers of retrieved oocytes, mature oocytes and fertilized oocytes were also significantly lower in the pioglitazone group (p<0.001 each), but there was no difference in the numbers of grade I or II embryos (4.5±2.0 vs. 4.1±2.1). The clinical pregnancy rate (44.8% vs. 41.8%) and live birth rate (44.2% vs. 34.9%) were higher in the pioglitazone than in the control group, but these differences were not statistically significant. Moreover, the incidence of severe ovarian hyperstimulation syndrome (OHSS) was lower in the pioglitazone group (2.3% vs. 9.3%), but the difference was not statistically significant.
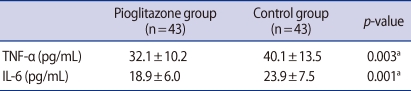
TNF-α and IL-6 concentrations in the FF of both groups are shown in Table 3. Both TNF-α (32.1±10.2 pg/mL vs. 40.1±13.5 pg/mL, p=0.003) and IL-6 (18.9±6.0 pg/mL vs. 23.9±7.5 pg/mL, p=0.001) concentrations were significantly lower in the pioglitazone than in the control group.
We have shown here that treatment with pioglitazone during 3 weeks of OC pretreatment and the period of ovarian stimulation reduced the concentrations of TNF-α and IL-6 in the FF of women with PCOS undergoing IVF. Although, in agreement with our previous results, we found that the number of retrieved oocytes was lower in the pioglitazone than in the control group, the development of grade I or II embryos was similar [4]. We also found that clinical pregnancy and live birth rates tended to be higher, and that serious complications such as OHSS tended to be lower, in the pioglitazone group.
TNF-α and IL-6 are important pro-inflammatory cytokines that may influence ovarian function and other reproductive processes [5]. These cytokines have been associated with the pathogenesis of endometriosis, a common benign gynecological disorder. For example, TNF-α concentrations in peritoneal fluid were found to be higher in women with than without endometriosis [9], suggesting that TNF-α may have a role in the establishment, maintenance and progression of endometriosis. IL-6 concentrations have also been reported to be higher in women with endometriosis [10], as well as in women with unexplained infertility [11]. Moreover, both TNF-α and IL-6 concentrations were found to be higher in PCOS patients than in body mass index-matched controls [6].
Due to the heterogeneous features of PCOS, its diagnosis and management remain unclear. Although the exact pathophysiology of PCOS is also unclear, hyperandrogenism and insulin resistance have been found to contribute to its etiology. Inflammatory cytokines closely linked to obesity and insulin resistance may be important in the pathogenesis of PCOS [12]. Women with PCOS were found to have chronic low grade inflammation, as determined by their concentrations of C-reactive protein [13], which is associated with risk factors of cardiovascular disease such as dyslipidemia, glucose intolerance, type 2 diabetes, hypertension and obesity [6]. Adipose tissue has been found to be the main source of TNF-α and IL-6 [14]. Infertile women with PCOS were found to have higher serum and FF concentrations of TNF-α and IL-6 than control women, suggesting that granulosa cells produce both cytokines [7]. Furthermore, a polymorphism in the IL-6 promoter region may be related to insulin resistance and metabolic abnormalities in PCOS [5].
Insulin-sensitizing agents have been investigated for their ability to manage insulin resistance, irregular menstruation, infertility and obesity in women with PCOS. The biguanide metformin has been recommended for PCOS patients with glucose intolerance, body mass index >35 kg/m2, or resistance to clomiphene citrate [4]. Another type of insulin sensitizer, troglitazone, a thiazolidinedione, has also been tested in these patients, but it was removed from the market because of serious liver toxicity. Two other thiazolidinediones, pioglitazone and rosiglitazone, are still being investigated in patients with PCOS; both agents have been found to improve menstrual irregularity and insulin sensitivity. Moreover, both have better safety profiles than troglitazone [1]. Despite their ability to enhance ovulation rate and improve fertility, they have not been tested in clinical trials of ovulation induction and IVF outcomes [15].
Our previous study was the first to assess the effect of pioglitazone on ovulation induction and IVF outcome in PCOS patients [4]. Measurements of the resistance index of the intraovarian stromal artery on the day of hCG injection showed that pioglitazone diminished blood flow in the ovarian stroma, enhanced the response to ovarian stimulation, and improved IVF outcomes in PCOS patients resistance to CC treatment. This study showed similar COS and IVF outcomes, except that the number of fertilized oocytes was significantly lower in the pioglitazone than in the control group, a difference that may be due to larger numbers of subjects in this study.
Thiazolidinediones bind to peroxisome proliferator-activated receptor gamma (PPAR-γ), which is located primarily in adipose tissue. Activation of this receptor decreases insulin resistance by enhancing fatty acid uptake into adipose tissue, producing more adiponectin and decreasing inflammatory mediators such as TNF-α and IL-6. PPAR-γ activation suppresses these inflammatory cytokines by negatively regulating macrophages in adipose tissue [1,16,17]. Moreover, the thiazolidinediones rosiglitazone was recently shown to significantly increase PPAR-γ mRNA and decrease TNF-α in human granulosa-lutein cells in vitro, and to decrease IL-6 secretion, although not significantly [18]. In ovaries, PPAR-γ is expressed more by granulosa cells than by theca cells and corpora lutea [19], findings consistent with our results. Pioglitazone may act on PPAR-γ present on granulosa cells and reduce TNF-α and IL-6 in FF. Cytokines within the ovary may act in an autocrine or paracrine manner, regulating follicular maturation and subsequent embryonic development [20]. Thus, by reducing TNF-α and IL-6 concentrations, pioglitazone may increase clinical pregnancy and live birth rates.
In conclusion, chronic low grade inflammation, represented by TNF-α and IL-6, may contribute to the pathophysiology of PCOS and may have a harmful effect on COS and IVF. Thus, relieving inflammation may be beneficial for PCOS patients undergoing IVF treatments. This study is the first to show that pioglitazone decreases FF concentrations of TNF-α and IL-6, resulting in better IVF outcomes. Further studies are needed to establish the effectiveness and long-term safety of pioglitazone treatment in women with PCOS.
Notes
References
1. Garruti G, Depalo R, Vita MG, Lorusso F, Giampetruzzi F, Damato AB, et al. Adipose tissue, metabolic syndrome and polycystic ovary syndrome: from pathophysiology to treatment. Reprod Biomed Online 2009;19:552-563.PMID: 19909598.


2. Stout DL, Fugate SE. Thiazolidinediones for treatment of polycystic ovary syndrome. Pharmacotherapy 2005;25:244-252.PMID: 15767238.


3. ACOG Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 108: Polycystic ovary syndrome. Obstet Gynecol 2009;114:936-949.PMID: 19888063.


4. Kim CH, Jeon GH, Kim SR, Kim SH, Chae HD, Kang BM. Effects of pioglitazone on ovarian stromal blood flow, ovarian stimulation, and in vitro fertilization outcome in patients with polycystic ovary syndrome. Fertil Steril 2010;94:236-241.PMID: 19324344.


5. Vural P, Değirmencioğlu S, Saral NY, Akgül C. Tumor necrosis factor alpha (-308), interleukin-6 (-174) and interleukin-10 (-1082) gene polymorphisms in polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 2010;150:61-65.PMID: 20189706.


6. Samy N, Hashim M, Sayed M, Said M. Clinical significance of inflammatory markers in polycystic ovary syndrome: their relationship to insulin resistance and body mass index. Dis Markers 2009;26:163-170.PMID: 19729797.



7. Amato G, Conte M, Mazziotti G, Lalli E, Vitolo G, Tucker AT, et al. Serum and follicular fluid cytokines in polycystic ovary syndrome during stimulated cycles. Obstet Gynecol 2003;101:1177-1182.PMID: 12798522.


8. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19-25.

9. Richter ON, Dorn C, Rosing B, Flaskamp C, Ulrich U. Tumor necrosis factor alpha secretion by peritoneal macrophages in patients with endometriosis. Arch Gynecol Obstet 2005;271:143-147.PMID: 14745563.


10. Bedaiwy MA, Falcone T, Sharma RK, Goldberg JM, Attaran M, Nelson DR, et al. Prediction of endometriosis with serum and peritoneal fluid markers: a prospective controlled trial. Hum Reprod 2002;17:426-431.PMID: 11821289.


11. Demir B, Guven S, Guven ES, Atamer Y, Gul T. Serum IL-6 level may have role in the pathophysiology of unexplained infertility. Am J Reprod Immunol 2009;62:261-267.PMID: 19706022.


12. Elkind-Hirsch KE. Thiazolidinediones for the therapeutic management of polycystic ovary syndrome: impact on metabolic and reproductive abnormalities. Treat Endocrinol 2006;5:171-187.PMID: 16677059.


13. Kelly CC, Lyall H, Petrie JR, Gould GW, Connell JM, Sattar N. Low grade chronic inflammation in women with polycystic ovarian syndrome. J Clin Endocrinol Metab 2001;86:2453-2455.PMID: 11397838.


14. Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol 2010;316:129-139.PMID: 19723556.


15. Katsiki N, Hatzitolios AI. Insulin-sensitizing agents in the treatment of polycystic ovary syndrome: an update. Curr Opin Obstet Gynecol 2010;22:466-476.PMID: 20724929.


16. Sharma AM, Staels B. Review: Peroxisome proliferator-activated receptor gamma and adipose tissue: understanding obesity-related changes in regulation of lipid and glucose metabolism. J Clin Endocrinol Metab 2007;92:386-395.PMID: 17148564.


17. Quinn CE, Hamilton PK, Lockhart CJ, McVeigh GE. Thiazolidinediones: effects on insulin resistance and the cardiovascular system. Br J Pharmacol 2008;153:636-645.PMID: 17906687.


18. Chen Q, Sun X, Chen J, Cheng L, Wang J, Wang Y, et al. Direct rosiglitazone action on steroidogenesis and proinflammatory factor production in human granulosa-lutein cells. Reprod Biol Endocrinol 2009;7:147PMID: 20003221.



19. Komar CM. Peroxisome proliferator-activated receptors (PPARs) and ovarian function: implications for regulating steroidogenesis, differentiation, and tissue remodeling. Reprod Biol Endocrinol 2005;3:41PMID: 16131403.



20. Qiao J, Feng HL. Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum Reprod Update 2011;17:17-33.PMID: 20639519.


-
METRICS

- Related articles in Clin Exp Reprod Med
-
Vitamin D deficiency in women with polycystic ovary syndrome2014 June;41(2)