|
 |
- Search
| Clin Exp Reprod Med > Volume 38(1); 2011 > Article |
Abstract
Objective
This study was carried out to compare the clinical outcome of elective single cleavage-embryo transfer (eSCET) to that of elective single blastocyst-embryo transfer (eSBET) in human IVF-ET.
Methods
This study was a retrospective study which analyzed for 614 women who visited the Daegu Maria Clinic from August 2008 to December 2009. All were under 37 years old and had more than 8 mm of endometrial thickness on the day of hCG administration and at least one good quality embryo on day 3. The eSCETs were performed on day 3 (n=450) and the eSBETs were conducted on day 5 (n=164).
Results
The numbers of retrieved oocytes, fertilized oocytes, and day 3 good quality embryos were significantly lower in the eSCET group (12.1±6.0, 8.2±4.6, and 4.2±3.1, respectively) compared to the eSBET group (16.7±7.2, 12.1±5.0, and 8.5±4.5, respectively; p<0.001). However, the clinical pregnancy, implantation, on-going pregnancy, and live birth rates of the eSCET group (46.7, 46.9, 40.0, and 36.7%, respectively) were not statistically different from those of the eSBET group (51.2, 51.8, 45.1, and 43.9%, respectively; p=0.318, 0.278, 0.254, and 0.103, respectively).
Conclusion
These results suggested that elective single embryo transfer should be performed regardless of the developmental stage to women less than 37 years old who had more than 8 mm of endometrial thickness on the hCG administration day and at least one good quality embryo on day 3 in order to reduce the twin pregnancy rate without reducing the whole pregnancy rate.
Multiple embryos have been transferred into the uterine cavity in order to maintain an acceptable pregnancy rate in IVF-ET programs. However, this had a high risk of complications including premature birth, low birth weight infants, intrauterine growth retardation, perinatal death, and cerebral palsy. It has been concluded that minimizing the number of transferred embryos is the best way to prevent those side effects. A 22% multiple birth rate was reported in the Europe in 2004 [1] and a 31% rate in the US in 2006 [2]. However, the multiple birth rate of Korea in 2006 was reported to be high as 33.8% [3]. To reduce multiple pregnancies, in 2008 the Korean Ministry of Health and Welfare recommended transferring one embryo on day 4 or 5 or two embryos on day 2 or 3 for patients who are less than 35 years of age with a good prognosis. A voluntary reduction in the number of transferred embryos to two from three resulted in an almost complete elimination of triplet pregnancies, while the pregnancy rate remained at approximately 35%--similar to that for three embryos, and the twin birth rate became 25% [4]. In addition, Kang et al. [5] reported that 60.7% of clinical pregnancies and 32.3% of multiple pregnancies were achieved for women less than 36 years old on day 3, resulting from two cleavage embryo transfer. In light of the above research, transferring just two embryos for patients with a good prognosis could not dramatically decrease multiple pregnancies.
One of the best ways to maintain a reasonable pregnancy rate while still reducing the number of multiple births in human IVF-ET would be to select and transfer a single embryo with the best quality of many embryos generated from a cohort. Actually, it was reported that the Nordic countries have successfully implemented a policy of single embryo transfer. In Sweden, for example, single embryo transfer was conducted for 70% of all patients [6]. An acceptable pregnancy rate must be maintained in order to perform single embryo transfer. Although most clinics have not performed single embryo transfer due to fears that the pregnancy rate could be lowered by reducing the number of transferred embryos, previous studies including more than 100 cycles showed that the clinical pregnancy and delivery rates of elective single cleavage-embryo transfer (eSCET) were similar to those of elective double cleavage-embryo transfer (eDCET) [5,7,8]. Likewise, it has been reported that the clinical pregnancy rate of elective single blastocyst-embryo transfer (eSBET) was similar to that of elective double blastocyst-embryo transfer (eDBET) on day 5 [9].
It would be difficult to accurately select the most competent of day 3 embryos for eSCET [10,11]. Bavister [12] reported that morula- or blastocyst-stage embryo transfer resulted in a significantly higher implantation rate than cleavage-stage embryo transfer in many mammalian species. Similarly, Gardner et al. [13] suggested that transferring blastocyst-stage embryos will enhance the likelihood of pregnancy. Also, some studies have shown that day 5 eSBET resulted in a significantly higher pregnancy rate [14-16] and delivery rate [15,16] compared with day 2 or 3 eSCET in selected groups. These results suggest that eSBET is one method that could reduce the multiple pregnancy rate while maintaining an acceptable pregnancy rate. However, Guerif et al. [14] showed that there was no statistically significant difference in the cumulative pregnancy and birth rates of eSCET and eSBET groups, including fresh and frozen embryo transfers. Transferring of blastocyst-stage embryos into the uterine cavity would be identical to the time of natural pregnancy [17]. The risk of aneuploidy in a blastocyst-stage embryo would be also lower than a cleavage-stage embryo [18,19]. However, there was no clear evidence that the blastocyst-stage embryo transfer resulted in a higher clinical outcome than the cleavage-stage embryo transfer [20]. Moreover, it has been reported that the implantation rate of the cleavage-stage embryo transfer was similar to that of the blastocyst-stage embryo transfer for patients with good prognosis [21]. It would show a higher cancellation rate due to failed embryo development to the blastocyst stage [22]. Also, it has been reported that transfer of blastocyst-stage embryo is related to a higher incidence of monozygotic twins [23], an altered sex ratio of births [24], and the possibilities of epigenetic modification [25]. Consequently, the optimal time for embryo transfer still remains controversial.
This study was carried out to compare the clinical outcome of eSCET to that of eSBET for patients with a good prognosis based on age, endometrial thickness, and quality of embryo in human IVF-ET.
This study was performed for 614 women who visited the Daegu Maria Clinic from August 2008 to December 2009. They were all under 37 years old and had more than 8 mm of endometrial thickness on the day of hCG administration, more than 2 fertilized embryos, and at least one good quality embryo on day 3. This study did not include oocyte donation cycles.
Ovarian stimulation was undertaken by using the GnRH agonist long protocol or GnRH antagonist protocol and recombinant FSH (Gonal-F; Merck Serono, Darmstadt, Germany). 10,000 IU of hCG (IVF-C; LG Life Science, Daejon, Korea) was injected when more than two follicles 17-18 mm in diameter were visible on ultrasonography. Oocyte retrieval was undertaken by transvaginal ultrasound-guided aspiration after 36 hours of hCG administration. The maturity of collected oocytes was determined depending on the features of corona radiata. The oocytes were washed in MRC#OW medium (Biosupply Co., Seoul, Korea) according to their maturity--mature, intermediate and immature, and then cultured in MRC#D01 medium (Biosupply Co.) until insemination or intracytoplasmic sperm injection (ICSI).
In vitro fertilization was induced by using conventional insemination or ICSI. Insemination was conducted in MRC#D01 medium (Biosupply Co.). After doing ICSI, oocyte culture was also performed in MRC#D01 medium (Biosupply Co.). Within the 16 to 18 hours, the oocyte with two pronuclei and a second polar body was regarded to be normally fertilized. The normally fertilized embryos were cultured until eSCET or eSBET was performed.
All the zygotes were co-cultured with autologous cumulus cells (ACC) in 20 µL of MRC#D16 medium (YS medium [26]; Biosupply Co.) containing autologous follicular fluid (AFF). Culture medium was exchanged for pre-equilibrated fresh medium every morning. AFF was collected from follicles that produced healthy mature oocytes with a clear corona radiata. AFF was used for culture after inactivation at 56℃ for 30 minutes and sterilization with a 0.22 µm filter in turns followed by centrifuging for 15 minutes at 3,000 rpm. ACCs were also prepared in a 5 µL micro droplet of an organ culture dish (353653, BD Falcon; Becton Dickinson Labware, Franklin Lakes, NJ, USA) under MRC#Oil (Biosupply Co.) by seeding its single cells followed by excising from a clear corona radiata of healthy cumuli and digesting with MRC#Hyase (Biosupply Co.). The first 48 hours of co-culture was supplemented with 10% AFF, and during the next 48 hours 20% AFF was added. In the middle of co-culture, only one embryo in the eSCET group was transferred on day 3 and the rest of the embryos were co-cultured until day 5 or 6. Similarly, only one embryo in the eSBET group was transferred on day 5. The surplus embryos that reached the blastocyst stage on day 5 or 6 were thereafter cryo-preserved by vitrifying one or two units per ampoule based on their quality.
The luteal phase was supported by administration of Crinone gel (90 mg, Merck Serono) and Utrogestan (100 mg, Lab. Besins Int., Paris, France) for 14 days after oocyte retrieval. The Crinon gel was taken once a day vaginally, while the Utrogestan was taken orally three times a day.
The quality of cleavage-stage embryos was assessed in the morning of day 3. A "good" quality day 3 embryo was defined as having more than 7-blastomeres of equal size and less than 20% fragmentation. The quality of blastocyst-stage embryos was assessed in the morning of day 5, which was categorized according to the criteria of Gardner and Schoolcraft [27]. The scoring system was based on the degree of expansion and hatching status of the blastocoel cavity (1-6), the size of the inner cell mass (A-C), and the development of the trophectoderm (A-C). It was standard that the blastocyst embryo transfer would be performed for women with more than 4 good embryos on day 3. However, most of the women with embryos produced by ICSI were excluded from this standard although they had several good quality embryos due to a fear of ET failure. The eSCET or eSBET was completed by transferring a single best embryo into the uterine cavity in each group.
After eSCET or eSBET, the surplus embryos were cocultured to day 5 or 6 with the ACC in the MRC#D16 containing 20% AFF. Only the normal embryos that reached the blastocyst stage were selected for cryopreservation based on the healthiness of trophectoderm cells and the size of the inner cell mass. The embryos were artificially shrunken for dehydration of the blastocoel using two 29-gauge needles, and then incubated for 5 minutes in MRC#CBS (Biosupply Co.). Vitrification of the embryos was carried out after exposure to equilibration solution for 1.5 minutes. The equilibration solution was MRC#CBS containing 20% (v/v) ethylene glycol (EG), while the vitrification solution was MRC#CBS supplemented with 40% (v/v) EG, 18% (w/v) ficoll, and 0.3 M sucrose. The equilibrated embryos were transferred into vitrification solution, loaded onto an EM grid, and directly plunged in liquid nitrogen within 30 seconds. Finally, the EM grid was moved in a cryovial previously submerged under liquid nitrogen. The cryovial was stored in liquid nitrogen.
Serum β-hCG concentration was measured 14 days after oocyte retrieval to verify pregnancy. Follow-up inspection was continuously conducted for women whose serum β-hCG concentration was positive. Clinical pregnancy was judged by observation of the gestational sac on vaginal ultrasonography after 6-7 weeks of gestation. A pregnancy with fetal heartbeat for longer than 10 weeks was also defined as an ongoing pregnancy. The implantation rate was indicated as the proportion of the gestational sacs to the transferred embryos.
Statistical analysis was performed with SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA) program, and the average value was expressed as the mean±standard deviation. For comparison of the continuous variables, the Student's t-test was used and for comparison of non continuous variable, the chi-square test was used. Results were considered statistically significant if p<0.05.
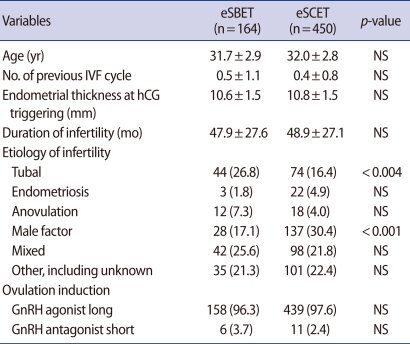
Of the women under 37 years old who visited the Daegu Maria Clinic for IVF-ET treatment from August 2008 to December 2009, 614 cases had an endometrial thickness was more than 8 mm on the day of hCG administration, more than 2 fertilized embryos, and at least one good quality embryo on day 3. eSBET was performed for 164 women on day 5, and eSCET was conducted for 450 women on day 3. The characteristics of the eSCET group compared to the eSBET group were shown in Table 1. There was no difference between the eSBET and eSCET groups in terms of the age of patients, number of previous IVF cycles, endometrial thickness at hCG administration, or duration of infertility. However, the tubal factor rate of the eSCET group was significantly lower than that of the eSBET group in etiology of infertility (16.4% vs. 26.8%; p<0.004), while the male factor rate of the eSCET group was significantly higher than that of the eSBET group (30.4% vs. 17.1%; p<0.001) (Table 1). On the other hand, there was no difference in the ovulation induction of the eSBET and eSCET groups. The GnRH agonist long protocol was applied to most of the women in each group as shown in Table 1 (eSBET, 96.3%; eSCET, 97.6%). Accordingly, the GnRH antagonist short protocol was used for a few women in each group (eSBET, 3.7%; eSCET, 2.4%).
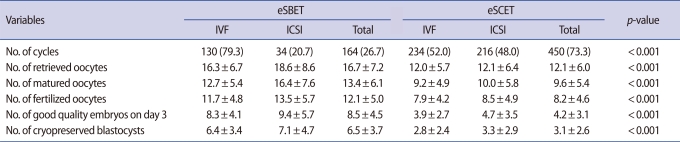
The proportion of ICSI attempts was significantly higher in the eSCET group compared with the eSBET group (48.0% vs. 20.7%; p<0.001), while that of conventional IVF was significantly lower in the eSCET group than in the eSBET group (52.0% vs. 79.3%; p<0.001) as shown in Table 2. Incidentally, there was no difference between the conventional IVF and ICSI rates in the eSCET group (52.0% vs. 48.0%), but those of the eSBET group were significantly different (79.3% vs. 20.7%; p<0.001). On the other hand, the numbers of retrieved and matured oocytes were significantly fewer in the eSCET group (12.1±6.0 and 9.6±5.4, respectively) compared to the eSBET group (16.7±7.2 and 13.4±6.1, respectively; p<0.001), but those within each group were the same as shown in Table 2. Similarly, the numbers of fertilized oocytes, good quality embryos, and cryopreserved blastocysts were significantly fewer in the eSCET group (8.2±4.6, 4.2±3.1, and 3.1±2.6, respectively) than those of the eSBET group (12.1±5.0, 8.5±4.5, and 6.5±3.7, respectively; p<0.001), but there was no difference between conventional IVF and ICSI cycles in those of each group (Table 2). Moreover, the fertilization, developmental competency, and cryopreservation rates had a tendency to become lower in the eSCET group (85.4%, 51.2%, and 43.1%, respectively) compared to the eSBET group (90.3%, 69.6%, and 58.6%, respectively), but there was no difference between conventional IVF and ICSI cycles in those of each group (data not shown).
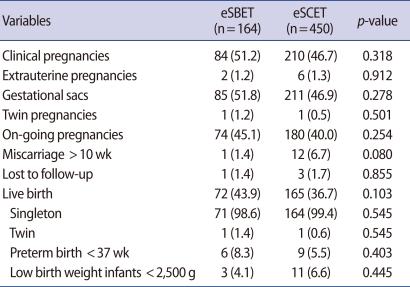
The clinical outcomes of the eSBET and eSCET groups are shown in Table 3. The clinical and ongoing pregnancy rates of the eSCET group (46.7% and 40.0%, respectively) showed a tendency to become lower than those of the eSBET group (51.2% and 45.1%, respectively), but there was no statistical significance between the two groups in those rates (p=0.318 and 0.254, respectively). The implantation rates of the eSBET and eSCET groups also did not show an appreciable difference (51.8% vs. 46.9%; p=0.278). The ectopic pregnancy rates were similar in the eSBET and eSCET groups, at 1.2% and 1.3%, respectively. Only one case of a pregnancy with monozygotic twins was found in each group (eSBET, 1.2% vs. eSCET, 0.5%; p=0.501). The abortion rate following 10-weeks showed a tendency to be higher in the eSCET group compared to the eSBET group, with no statistical difference (6.7% vs. 1.4%; p=0.080). The live birth rate of the eSCET group was statistically similar to that of the eSBET group (36.7% vs. 43.9%; p=0.103). Moreover, the preterm (before 37 weeks) and low weight (less than 2,500 g) birth rates of the eSCET group (5.5% and 6.6%, respectively) were statistically identical to those of the eSBET group (8.3% and 4.1%). Finally, most of the live birth babies were singletons (eSBET, 98.6% vs. eSCET, 99.4%), which was similar for the two groups.
The present study showed that the clinical pregnancy, implantation, on-going pregnancy, and live birth rates of the eSCET group were not statistically different from those of the eSBET group although the numbers of retrieved oocytes, fertilized oocytes, and day 3 good quality embryos were significantly lower in the eSCET group than in the eSBET group (Table 3). Only one case of a multiple pregnancy with monozygotic twins was found in each group, while the live birth rates tended to be lower in the eSCET group than the eSBET group but there was no statistical significance between the two groups. The preterm and low weight birth rates also showed a tendency to be lower in the eSCET group than the eSBET group, with no statistical difference between the two groups (Table 3). However, Papanikolaou et al. [15], Zech et al. [16], and Guerif et al. [14] had reported that the pregnancy and birth rates were lower in those undergoing eSCET than in those undergoing eSBET. Taken together, it was suggested that the clinical outcome of the eSCET group should be similar to that of the eSBET group if women in whom a single embryo will be replaced are selected on the basis of several factors, including age, endometrial thickness, embryo number, and day 3 embryo qualities.
A weakness of the present study was that it was a retrospective study. Thus, the proportion of tubal factor among the etiology of infertility was significantly lower in the eSCET group than the eSBET group (p<0.004), whereas the proportion of male factor was significantly higher in the eSCET group than the eSBET group (p<0.001) (Table 1). Also, the eSBET was actually assigned to the women with full procurement of good quality embryos on the 3rd day to prevent the cancellation of blastula transfer among the embryos fertilized by conventional insemination. Thus, it was concluded that the number of retrieved oocytes, normal zygotes and day 3 good quality embryos were much higher in the eSBET group than the eSCET group (Table 2). Meanwhile, the attempt ratio of ICSI was significantly higher in the eSCET group than the eSBET group (p<0.001), resulting from the report of Miller and Smith [28] that the blastula formation rate was lower in the oocytes fertilized by ICSI than those fertilized by conventional insemination. It was also concluded that more ICSI cycles were composed in the eSCET group as the embryos derived from ICSI were transferred as close to day 3 as possible though there were a large number of good quality embryos. In the present study, the blastula rates of embryos derived from ICSI could not be demonstrated because they were not assessed. It was found that the clinical pregnancy rate derived from ICSI was similar to that of conventional insemination in the eSBET group (50% vs. 51.5%). On the other hand, it was also observed that the clinical pregnancy rates between ICSI and conventional insemination of the eSCET group were similar (46.3% vs. 47.0%). Likewise, the birth rates derived from ICSI between the eSBET and eSCET groups were 44.1% (15/34) and 36.6% (79/216), respectively, which were not different from 43.9% (57/130) and 36.8% (86/234), respectively, of conventional insemination in each group. Accordingly, it was concluded that although there was a significant difference between the two groups in the ICSI usage ratio, fertilization methods were careless about the clinical pregnancy and birth rates in both groups.
Previous studies have found that a higher implantation rate could be achieved by transferring blastulas than by transferring cleavage-stage embryos for several reasons. Magli et al. [18] reported that approximately 51% of "top quality" day 3 embryos had aneuploidy, which was higher than 35% of blastocysts. They mentioned that the embryos with genetic abnormalities have difficulty developing to the blastocyst stage, so that transfer of blastulas will guide to selecting better quality embryos compared to cleavage-stage embryos [18,19]. Also, Gardner et al. [17] reported that transferring the cleavage-stage embryos to the uterine cavity can damage the developmental competence of embryos, resulting from the stress of metabolism as the oviduct and uterus provide different nutritional environments to embryos. Based on the given reasons, previous studies on the eSCET and eSBET have reported that the clinical pregnancy and birth rates of those undergoing eSBET were significantly higher than those undergoing eSCET [14-16]. However, the present study showed that the clinical pregnancy and birth rates of the eSBET group had a tendency to be higher with eSCET but there was no statistical difference between the two groups (Table 3). It was concluded that the results were attributed to selection of women less than 37 years old who possessed more than 8 mm of endometrial thickness on hCG administration day, more than two fertilized embryos, and at least one good quality embryo on day 3.
When one embryo is transferred in IVF-ET programs, monozygotic pregnancies rarely occurred, while dizygotic twin pregnancies were completely prevented. The frequency of monozygotic twin pregnancies has been reported to be approximately 2-5% following eSBET. In addition, Papanikolaou et al. [15] reported that monozygotic twin pregnancies were 2% in the eSCET group, while there were no cases of monozygotic twins in the eSBET group. In the present study, the proportion of monozygotic twin pregnancies was similar between the eSBET and eSCET groups (0.6% vs. 0.5%). These were identical to the result of natural pregnancy, which was 1/330 [29]. On the other hand, monozygotic pregnancies were reported to be increased by transferring blastulas rather than cleavage-stage embryos [23], resulting in higher perinatal morbidity and mortality compared to dizygotic twins [30]. Guerif et al. [14] reported that the monozygotic twin birth rate was slightly higher in the eSBET group than the eSCET group (3.8% vs. 1.6%) but there was no statistical significance among any of the groups [14]. da Costa et al. [31] reported that the monozygotic twin pregnancies were increased by transfer of the blastula, and the reason might be due to more damage and hardening of the zona pellucida from in vitro culture environment compared to cleavage-stage embryos. Therefore, it is considered to be necessary for further research about the correlation between blastula transfer and monozygotic twins.
Ectopic pregnancies have been reported to occur in approximately 2-5% of clinical pregnancies after IVF-ET [32,33]. Stimulations caused by collecting the ovum, plus culture medium injected for transferring embryos and methods of transferring can be major factors of ectopic pregnancies. It is considered that uterine contraction make transferred embryos move toward the fallopian tube, caused by those factors. Fanchin et al. [34] indicated that ectopic pregnancies should be decreased by transfer of blastulas compared to cleavage-stage embryos as uterine contractility will gradually decline according to the time following oocyte retrieval. Schoolcraft et al. [35] also indicated that the diameter of the blastula is larger than that of the cleavage-stage embryo, so that blastula transfer should reduce the movement of the embryo to the fallopian tube. In the present study, the ectopic pregnancies of eSCET group were 2.8% (6/216), which was identical to 2.3% (2/86) of the eSBET group. On the other hand, there was a case in which blastula transfer increased ectopic pregnancy compared to cleavage-stage embryo transfer [36].
In 2009, the National Statistical Office announced that the preterm birth and low weight birth rates of singleton pregnancies were 2.2% and 3.5%, respectively, whereas those of multiple pregnancies were 28.9% and 54.4%, respectively. In the present study, the preterm birth and low weight birth rates derived from single embryo transfer were 6.0% and 5.9%, respectively, which was to exclude 2 cases of monozygotic twin pregnancies (there were 50% of preterm and low weight birth among these). These results indicated that the premature birth and low weight birth infant should clearly be increased by multiple pregnancies. Thus, Daegu Maria Clinic has gradually reduced the number of transferred embryos to minimize multiple pregnancies in since 2007. In 2010, only an elective single embryo was transferred to about 55% of women. In fact, most of the women refused this method for fear that the pregnancy rate could be reduced. However, once the resistance had been almost eliminated as they understood the fact that the overall pregnancy rate had no difference: although only single embryo is transferred, the surplus embryos are stored, and if more than 2 embryos are transferred to women, complications can occur to women as a result of multiple pregnancies. Currently, the average number of embryos transferred at Daegu Maria Clinic was about 1.4 units, and vitrification of the surplus embryos was undertaken for about 68% of women.
In conclusion, these results suggest that elective single embryo should be transferred regardless of the developmental stage to women less than 37 years old who had more than 8 mm of endometrial thickness on hCG administration day and at least one good quality embryo on day 3 to reduce the twin pregnancy rate, without reducing the whole pregnancy rate. It is concluded that elective single embryo transfer will dramatically improve the cumulative pregnancy rate because enough surplus embryos can be preserved for many patients.
References
1. Andersen AN, Goossens V, Ferraretti AP, Bhattacharya S, Felberbaum R, de Mouzon J, et al. Assisted reproductive technology in Europe, 2004: results generated from European registers by ESHRE. Hum Reprod 2008;23:756-771.PMID: 18281243.


2. Centers for Disease Control and Prevention. American Society for Reproductive Medicine. Society for Assisted Reproductive Technology. 2006 Assisted reproductive technology success rates: national summary and fertility clinic reports. 2008. Atlanta (GA): Department of Health and Human Services, Centers for Disease Control and Prevention.
3. ART committee; Korean Society of Obstetrics and Gynecology. Current status of assisted reproductive technology in Korea, 2006. Korean J Obstet Gynecol 2009;52:1212-1238.
4. 2004 Official statistics of Sweden [Internet]. Socialstyrelsen. 2010. cited 2010 Dec 2. Stockholm, SE: National Board of Health and Welfare; Available from: http://www.socialstyrelsen.se/
5. Kang SM, Lee SW, Jeong HJ, Chae SJ, Yoon SH, Lim JH, et al. Clinical outcome of elective single embryo transfer compared to elective double embryo transfer performed at the cleavage stage. Korean J Reprod Med 2010;37:349-359.
6. Nygren KG. Single embryo transfer: the role of natural cycle/minimal stimulation IVF in the future. Reprod Biomed Online 2007;14:626-627.PMID: 17509206.


7. De Sutter P, Van der Elst J, Coetsier T, Dhont M. Single embryo transfer and multiple pregnancy rate reduction in IVF/ICSI: a 5-year appraisal. Reprod Biomed Online 2003;6:464-469.PMID: 12831596.


8. Tiitinen A, Unkila-Kallio L, Halttunen M, Hyden-Granskog C. Impact of elective single embryo transfer on the twin pregnancy rate. Hum Reprod 2003;18:1449-1453.PMID: 12832371.


9. Henman M, Catt JW, Wood T, Bowman MC, de Boer KA, Jansen RP. Elective transfer of single fresh blastocysts and later transfer of cryostored blastocysts reduces the twin pregnancy rate and can improve the in vitro fertilization live birth rate in younger women. Fertil Steril 2005;84:1620-1627.PMID: 16359955.

10. Milki AA, Hinckley MD, Gebhardt J, Dasig D, Westphal LM, Behr B. Accuracy of day 3 criteria for selecting the best embryos. Fertil Steril 2002;77:1191-1195.PMID: 12057727.


11. Rijnders PM, Jansen CA. The predictive value of day 3 embryo morphology regarding blastocyst formation, pregnancy and implantation rate after day 5 transfer following in-vitro fertilization or intracytoplasmic sperm injection. Hum Reprod 1998;13:2869-2873.PMID: 9804247.


12. Bavister BD. Culture of preimplantation embryos: facts and artifacts. Hum Reprod Update 1995;1:91-148.PMID: 15726768.


13. Gardner DK, Surrey E, Minjarez D, Leitz A, Stevens J, Schoolcraft WB. Single blastocyst transfer: a prospective randomized trial. Fertil Steril 2004;81:551-555.PMID: 15037401.


14. Guerif F, Lemseffer M, Bidault R, Gasnier O, Saussereau MH, Cadoret V, et al. Single Day 2 embryo versus blastocyst-stage transfer: a prospective study integrating fresh and frozen embryo transfers. Hum Reprod 2009;24:1051-1058.PMID: 19218575.


15. Papanikolaou EG, Camus M, Kolibianakis EM, Van Landuyt L, Van Steirteghem A, Devroey P. In vitro fertilization with single blastocyst-stage versus single cleavage-stage embryos. N Engl J Med 2006;354:1139-1146.PMID: 16540614.


16. Zech NH, Lejeune B, Puissant F, Vanderzwalmen S, Zech H, Vanderzwalmen P. Prospective evaluation of the optimal time for selecting a single embryo for transfer: day 3 versus day 5. Fertil Steril 2007;88:244-246.PMID: 17292362.


17. Gardner DK, Schoolcraft WB, Wagley L, Schlenker T, Stevens J, Hesla J. A prospective randomized trial of blastocyst culture and transfer in in-vitro fertilization. Hum Reprod 1998;13:3434-3440.PMID: 9886530.


18. Magli MC, Jones GM, Gras L, Gianaroli L, Korman I, Trounson AO. Chromosome mosaicism in day 3 aneuploid embryos that develop to morphologically normal blastocysts in vitro. Hum Reprod 2000;15:1781-1786.PMID: 10920103.


19. Staessen C, Platteau P, Van Assche E, Michiels A, Tournaye H, Camus M, et al. Comparison of blastocyst transfer with or without preimplantation genetic diagnosis for aneuploidy screening in couples with advanced maternal age: a prospective randomized controlled trial. Hum Reprod 2004;19:2849-2858.PMID: 15471934.


20. Blake DA, Proctor M, Johnson NP. The merits of blastocyst versus cleavage stage embryo transfer: a Cochrane review. Hum Reprod 2004;19:795-807.PMID: 15033948.


21. Rienzi L, Ubaldi F, Iacobelli M, Ferrero S, Minasi MG, Martinez F, et al. Day 3 embryo transfer with combined evaluation at the pronuclear and cleavage stages compares favourably with day 5 blastocyst transfer. Hum Reprod 2002;17:1852-1855.PMID: 12093851.


22. Tsirigotis M. Blastocyst stage transfer: pitfalls and benefits. Too soon to abandon current practice? Hum Reprod 1998;13:3285-3289.PMID: 9886498.


23. Behr B, Fisch JD, Racowsky C, Miller K, Pool TB, Milki AA. Blastocyst-ET and monozygotic twinning. J Assist Reprod Genet 2000;17:349-351.PMID: 11042833.



24. Ménézo YJ, Chouteau J, Torelló J, Girard A, Veiga A. Birth weight and sex ratio after transfer at the blastocyst stage in humans. Fertil Steril 1999;72:221-224.PMID: 10438983.


25. Maher ER, Brueton LA, Bowdin SC, Luharia A, Cooper W, Cole TR, et al. Beckwith-Wiedemann syndrome and assisted reproduction technology (ART). J Med Genet 2003;40:62-64.PMID: 12525545.



26. Yoon HG, Yoon SH, Son WY, Kim JG, Im KS, Lim JH. Alternative embryo transfer on day 3 or day 5 for reducing the risk of multiple gestations. J Assist Reprod Genet 2001;18:262-267.PMID: 11464577.



27. Gardner DK, Schoolcraft WB. In : Jansen R, Mortimer D. In vitro culture of human blastocysts. In: Towards reproductive certainty: infertility and genetics beyond 1999 1999;In: The plenary proceedings the 11th World Congress on In Vitro Fertilization and Human Reproductive Genetics; New York, Parthenon Pub. Group. pp 378-388.
28. Miller JE, Smith TT. The effect of intracytoplasmic sperm injection and semen parameters on blastocyst development in vitro. Hum Reprod 2001;16:918-924.PMID: 11331638.


30. Lopriore E, Stroeken H, Sueters M, Meerman RJ, Walther F, Vandenbussche F. Term perinatal mortality and morbidity in monochorionic and dichorionic twin pregnancies: a retrospective study. Acta Obstet Gynecol Scand 2008;87:541-545.PMID: 18446538.


31. da Costa ALE, Abdelmassih S, de Oliveira FG, Abdelmassih V, Abdelmassih R, Nagy ZP, et al. Monozygotic twins and transfer at the blastocyst stage after ICSI. Hum Reprod 2001;16:333-336.PMID: 11157829.


32. Milki AA, Jun SH. Ectopic pregnancy rates with day 3 versus day 5 embryo transfer: a retrospective analysis. BMC Pregnancy Childbirth 2003;3:7PMID: 14604439.



33. Strandell A, Thorburn J, Hamberger L. Risk factors for ectopic pregnancy in assisted reproduction. Fertil Steril 1999;71:282-286.PMID: 9988399.


34. Fanchin R, Ayoubi JM, Righini C, Olivennes F, Schönauer LM, Frydman R. Uterine contractility decreases at the time of blastocyst transfers. Hum Reprod 2001;16:1115-1119.PMID: 11387279.


35. Schoolcraft WB, Surrey ES, Gardner DK. Embryo transfer: techniques and variables affecting success. Fertil Steril 2001;76:863-870.PMID: 11704102.


36. Keegan DA, Morelli SS, Noyes N, Flisser ED, Berkeley AS, Grifo JA. Low ectopic pregnancy rates after in vitro fertilization: do practice habits matter? Fertil Steril 2007;88:734-736.PMID: 17316634.