|
 |
- Search
| Clin Exp Reprod Med > Volume 38(4); 2011 > Article |
Abstract
Objective
During stimulated IVF cycles, up to 15% of oocytes are recovered as immature. The purpose of this study was to investigate the trend of oocyte maturity in repeated ovarian stimulation for IVF.
Methods
One hundred forty-eight patients were selected who underwent two consecutive IVF cycles using same stimulation protocol during 2008 to 2010. Ovarian stimulation was performed with FSH and human menopausal gonadotropin and flexible GnRH antagonist protocol in both cycles. Oocyte maturity was assessed according to presence of germinal vesicle (GV) and the first polar body. Immature oocyte was defined as GV stage or metaphase I oocyte (GV breakdown with no visible polar body) and cultured up to 48 hours. If matured, they were fertilized with ICSI.
Results
Percentages of immature oocytes were 30.8% and 32.9% (p=0.466) and IVM rates of immature oocytes were 36.2% and 25.7% (p=0.077), respectively. A significant correlation was noted between percentage of immature oocytes in the two cycles (R=0.178, p=0.03). Women with >40% immaturity in both cycles (n=21) showed lower fertilization rate of in vivo matured oocytes (56.4% vs. 72.0%, p=0.005) and lower pregnancy rate (19.0% vs. 27.1%, p=0.454) after the second cycle when compared with women with <40% immaturity (n=70). In both groups, female age, number of total retrieved oocyte and embryos transferred were similar.
Oocyte maturation is a process where the oocyte is escaped from the meiotic arrest and advances from prophase I to metaphase II [1]. Oocytes are maintained in meiotic arrest by inhibitory environment and the LH surge induces oocyte maturation to release the first polar body (PB) [2]. This meiotic progression is precisely regulated by various components.
In IVF cycle, multiple follicles are growing by administration of exogenous gonadotropins and oocytes are finally maturated by injection of hCG. In the preovulatory follicles, oocytes are arrested in prophase I and oocyte meiosis is resumed just after administration of hCG [3]. Although hCG is exposed for the sufficient time to induce the ovulation [4], the retrieved oocytes sometimes fail to resume meiosis in vivo. About 5-7% of oocytes retrieved are immature at the germinal vesicle (GV) stage, which were required further IVM process [5]. In IVF cycles using GnRH antagonist, up to 15% of oocytes are recovered as immature [6]. Immature oocytes can be fertilized after IVM, but generally resulting lower pregnancy rate than those matured in vivo. Therefore, obtaining higher proportion of in vivo matured oocyte is essential component for achieving good clinical pregnancy rate in stimulated IVF cycle.
The mechanisms to yield immature oocytes are not fully understood. Using FSH alone rather than FSH and LH for ovarian stimulation [7], GnRH antagonist rather than GnRH agonist [8] and a short GnRH agonist protocol rather than long GnRH agonist protocol [9] were reported to be related with immature oocytes. However, there have been no studies about the trend of oocyte maturity in the same person. We investigated the trend of immature oocytes retrieval on the assumption that immature oocytes would be retrieved repetitively in the consecutive IVF cycles in same women.
We retrospectively analyzed the data from 148 patients who underwent two consecutive IVF cycles using same stimulation protocol between 2008 and 2010. The study was approved by the Institutional Review Board of Maria Hospital. Exogenous gonadotropins, FSH (Gonal-f; Serono, Geneva, Switzerland) and human menopausal gonadotropin (hMG) (Menopur, Ferring, Denmark) and, GnRH antagonist (Cetrotide; Serono) were used for ovarian stimulation in both cycles with same protocol. Ovulation was induced by recombinant hCG 250 µg (Ovidrel; Serono) when a leading follicle had achieved a diameter of 18 mm or more. About 36 hours after administration of hCG, oocyte retrieval was performed under ultrasound guidance. All antral follicles were aspirated, including the small ones. Maturity of oocytes was classified according to nuclear status: presence of the first PB or possession of an intact GV. Immature oocytes defined as GV stage or metaphase I oocyte (GV breakdown with no visible PB) were then cultured up to 48 hours using maturation medium. After maturation, oocytes were denuded of the granulose cells and fertilized by ICSI. In vivo matured oocytes were also fertilized with conventional insemination or ICSI as appropriate. Embryo transfer was performed 3 days after oocyte retrieval. Clinical pregnancy was defined as the presence of intrauterine gestational sac by ultrasound at 6-7 weeks gestation.
The data were analyzed using the SPSS (SPSS Inc., Chicago, IL, USA) for Windows program and are presented as means±SD or %. Statistical analysis was performed by using Student's t-test or the Mann-Whitney U test for continuous variables and by using χ2-test for categorical variables. Correlations between different parameters were determined by univariate correlation analysis and are expressed as Spearman's correlation coefficients. A p-value of <0.05 was considered to be significant.
The mean age of total 148 women was 36.3±4.3 years and mean interval between the first and the second cycle was 167.0±117.1 days. The infertility factors of the subjects were identified as female factor (46.0%), unknown (25.0%), both factor (15.5%), and male factor (13.5%). The specific causes of infertility among those with the female and both factors were as follows: diminished ovarian reserve (42.8%), tubal obstruction (29.7%), endometriosis (16.5%), myoma (5.5%), thin endometrium (3.3%), and polycystic ovary syndrome (2.2%).
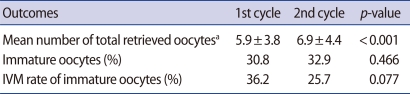
The outcome parameters of the first and second ovarian stimulation cycles are shown in Table 1. Mean number of total retrieved oocytes was 5.9±3.8 and 6.9±4.4 in the first and second cycles, respectively. Percentage of immature oocytes was 30.8% and 32.9% and IVM rate was 36.2% and 25.7%. Mean number of total retrieved oocytes was statistically higher in second cycle than first cycle. However, there was no significant difference between two groups in the rates of immature oocytes and IVM. Percentages of immature oocytes between the two cycles showed a significant correlation (R=0.178, p=0.03) (Figure 1).
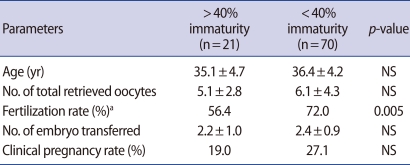
Patients were divided into two groups according to percentage of immature oocytes (Table 2). Women with >40% immaturity in both cycles showed lower fertilization rate of in vivo matured oocytes and lower pregnancy rate after the second cycle when compared with those having <40% immaturity. Age, number of total retrieved oocytes and transferred embryos were not significantly different in both groups.
Maturation of oocyte is an essential factor for successful fertilization. Bar-Ami et al. [10] reported that meiotically incompetent oocytes are retrieved in 8.6% to 15.2% of all infertility patients and also demonstrated that if >25% of the oocytes were immature, then successful fertilization and clinical pregnancy were greatly reduced [10]. The other studies also demonstrated that a positive correlation exists between the degree of nuclear maturation and their potential for fertilization in vitro [11,12]. Laufer et al. [13] observed a high fertilization rate of denuded oocytes which had a PB at the time of insemination, whereas oocytes at the GV or germinal vesicle breakdown stage failed to fertilize. In this study, we demonstrated that immature oocytes are retrieved in those with poor quality of follicular cohort and retrieved repeatedly in the repeat ovarian stimulation cycles. Moreover, the patients with >40% immaturity in both cycles showed lower fertilization rate.
The process of oocyte maturation is complex and not yet fully understood [14]. The meiotic division of the mammalian oocyte is under several stop/go control and influenced by various components of the oocyte and the follicle [10]. It starts before or after birth and restricted in status of meiotic arrest at the diplotene stage by an inhibitory follicular environment [15] and the LH surge lifts this restriction and stimulates the maturation of oocytes to the first PB stage [2]. Usually the resumption of meiosis takes place within 18 hours and achievement of second metaphase within 28-38 hours after LH surge [3]. Although hCG exposure is maintained for 36 hours which length of time is sufficient to induce the ovulatory process [4], sometimes the retrieved oocytes remained the status of meiotic arrest.
Levan et al. [16] suggested that failure of maturation in vivo may due to one of the following three causes: 1) absent or incomplete LH effect; 2) derangement in the signaling mechanism from the surrounding cumulus cells; and 3) intrinsic oocyte factors. Abnormal or insufficient LH effect involves the following mechanisms: inadequate timing of the hCG administration, lack of LH activity (hCG batch problem, i.e., inactive isoform), dysfunctional or insufficient LH receptors, problem in hormonal delivery [16,17].
Several strategies have been suggested for dealing with the problem of repeated retrieval of immature oocytes. For example, an extended in vivo period of follicular growth to a follicular size of 22-23 mm, an increase of hCG dose, an extended in vivo hCG interval, and modified natural cycle protocols have been recommended [18]. The results of the most trial have been disappointing and there are no available therapeutic approaches except oocyte donation. Hourvitz et al. [18] recommended that IVM treatment should be considered for women with repeated IVF failure. Dysfunction of the cumulus-oocyte complex might result in failure of the oocyte to resume meiosis. Therefore, early oocyte pickup and replacement of the natural inadequate environment with the more favorable medium might help the oocytes mature [18]. However, IVM is at the experimental level, and warrants further study.
Repeated retrieval of immature oocytes in IVF is associated with poor prognosis and difficult challenge to the physician solve. However, there have been no studies about the repetitiveness of immature oocytes. To our best knowledge, this is the first study about the oocyte maturity in repeated ovarian stimulation. In this study, we demonstrated that the immature oocyte tended to be retrieved repetitively in consecutive IVF cycles. Women with repeatedly higher oocyte immaturity had low potential for fertilization, which suggests poor quality of their follicular cohort.
This study had a number of limitations. First, oocyte maturation is influenced by the factors, such as duration of COH, difference between clinicians, dose of gonadotropin. However, these confounding factors are not considered in our study. Second, the solutions to reduce immature oocytes have not been suggested.
In conclusion, the immature oocyte tended to be retrieved repetitively in same women. Prediction of percentage of immature oocytes allows appropriate counseling and modification of an individual's treatment. Further studies are needed to solve this problem and improve the outcome of IVF in those with repeated immature oocyte retrieval.
References
1. Jamnongjit M, Hammes SR. Oocyte maturation: the coming of age of a germ cell. Semin Reprod Med 2005;23:234-241.PMID: 16059829.



2. Dekel N, Sherizly I, Phillips DM, Nimrod A, Zilberstein M, Naor Z. Characterization of the maturational changes induced by a GnRH analogue in the rat ovarian follicle. J Reprod Fertil 1985;75:461-466.PMID: 2999382.


3. Seibel MM, Smith DM, Levesque L, Borten M, Taymor ML. The temporal relationship between the luteinizing hormone surge and human oocyte maturation. Am J Obstet Gynecol 1982;142:568-572.PMID: 7058861.


4. Testart J, Frydman R, Feinstein MC, Thebault A, Roger M, Scholler R. Interpretation of plasma luteinizing hormone assay for the collection of mature oocytes from women: definition of a luteinizing hormone surge-initiating rise. Fertil Steril 1981;36:50-54.PMID: 7250407.


5. De Vos A, Van de Velde H, Joris H, Van Steirteghem A. In-vitro matured metaphase-I oocytes have a lower fertilization rate but similar embryo quality as mature metaphase-II oocytes after intracytoplasmic sperm injection. Hum Reprod 1999;14:1859-1863.PMID: 10402405.


6. Jee BC, Han SH, Moon JH, Suh CS, Kim SH. Seoul National University College of Medicine Assisted Reproductive Technology (SMART) Study Group. Influence of well defined protein source on in vitro maturation of human oocyte: human follicular fluid versus human serum albumin. Fertil Steril 2008;89:348-352.PMID: 17482173.


7. Huddleston HG, Jackson KV, Doyle JO, Racowsky C. hMG increases the yield of mature oocytes and excellent-quality embryos in patients with a previous cycle having a high incidence of oocyte immaturity. Fertil Steril 2009;92:946-949.PMID: 19356754.


8. Nogueira D, Friedler S, Schachter M, Raziel A, Ron-El R, Smitz J. Oocyte maturity and preimplantation development in relation to follicle diameter in gonadotropin-releasing hormone agonist or antagonist treatments. Fertil Steril 2006;85:578-583.PMID: 16500322.


9. Greenblatt EM, Meriano JS, Casper RF. Type of stimulation protocol affects oocyte maturity, fertilization rate, and cleavage rate after intracytoplasmic sperm injection. Fertil Steril 1995;64:557-563.PMID: 7641910.


10. Bar-Ami S, Zlotkin E, Brandes JM, Itskovitz-Eldor J. Failure of meiotic competence in human oocytes. Biol Reprod 1994;50:1100-1107.PMID: 8025167.


11. Lanzendorf SE, Zelinski-Wooten MB, Stouffer RL, Wolf DP. Maturity at collection and the developmental potential of rhesus monkey oocytes. Biol Reprod 1990;42:703-711.PMID: 2189504.


12. Gwatkin RB, Conover JC, Collins RL, Quigley MM. Failed fertilization in human in vitro fertilization analyzed with the deoxyribonucleic acid-specific fluorochrome Hoechst 33342. Am J Obstet Gynecol 1989;160:31-35.PMID: 2463758.


13. Laufer N, Tarlatzis BC, DeCherney AH, Masters JT, Haseltine FP, MacLusky N, et al. Asynchrony between human cumulus-corona cell complex and oocyte maturation after human menopausal gonadotropin treatment for in vitro fertilization. Fertil Steril 1984;42:366-372.PMID: 6432586.


14. Eichenlaub-Ritter U, Peschke M. Expression in in-vivo and in-vitro growing and maturing oocytes: focus on regulation of expression at the translational level. Hum Reprod Update 2002;8:21-41.PMID: 11866238.


15. Veeck LL. Extracorporeal maturation: Norfolk, 1984. Ann N Y Acad Sci 1985;442:357-367.PMID: 3925842.


16. Levran D, Farhi J, Nahum H, Glezerman M, Weissman A. Maturation arrest of human oocytes as a cause of infertility: case report. Hum Reprod 2002;17:1604-1609.PMID: 12042285.


17. Salha O, Abusheikha N, Sharma V. Dynamics of human follicular growth and in-vitro oocyte maturation. Hum Reprod Update 1998;4:816-832.PMID: 10098473.


18. Hourvitz A, Maman E, Brengauz M, Machtinger R, Dor J. In vitro maturation for patients with repeated in vitro fertilization failure due to "oocyte maturation abnormalities.". Fertil Steril 2010;94:496-501.PMID: 19589517.