|
 |
- Search
| Clin Exp Reprod Med > Volume 38(4); 2011 > Article |
Abstract
Objective
This study was performed to compare the efficiency of slow freezing and vitrification based on survival, development to blastocysts, and cell numbers of blastocysts. Changes in embryonic gene expression in fresh and frozen-thawed embryos were also examined.
Methods
Eight-cell stage embryos were collected from superovulated female BDF1 mice. The collected embryos were randomly divided into three groups. One group was maintained as fresh controls (n=42), one was frozen by slow freezing (n=43), and one was cooled by vitrification (n=43). After thawing or cooling, survival rates, development to blastocyst, and cell numbers and inner cell mass (ICM) cell numbers of blastocysts were compared with those of the control group. The expressions of eight genes (Rbm3, Birc5, Sod1, Sod2, Cirbp, Caspase3, Trp53, Hsp70.1) were examined by real time-quantitative polymerase chain reaction in the fresh and frozen-thawed embryos.
Results
There were no significant differences in the slow freezing and vitrification groups' survival rate after thawing (88.4% vs. 88.4%), development to blastocyst (100% vs. 97.4%), cell numbers (107.0┬▒21.0 vs. 115.0┬▒19.7), or ICM cell numbers of blastocysts (11.3┬▒5.2 vs. 11.1┬▒3.7). Cell numbers of blastocysts were significantly (p<0.05) lower in the frozen-thawed embryos than the fresh embryos. There were no significant differences in the slow freezing and the vitrification groups' expressions of the eight genes. The expressions of CirbP and Hsp70.1 were higher in the frozen-thawed embryos than in the fresh embryos but there were no significant differences.
After the birth of the first 'test-tube baby' [1], assisted reproductive technologies (ART) have advanced remarkably. Among these advancements, embryo cryopreservation has become a pivotal part of human IVF-ET, with frozen-thawed embryo transfer constituting approximately 20% of all ET worldwide [2]. Embryo cryopreservation has several advantages for increasing pregnancy rates in human IVF and ET programs. Cryopreservation of supernumerary embryos obtained from a single IVF cycle allows single or double ET and multiple ETs, thus preventing multiple pregnancies and enhancing cumulative pregnancy rates. Concomitantly, embryo cryopreservation can be used to postpone embryo transfer in patients who are at high risk of ovarian hyperstimulation syndrome and preserve fertility options for patients preparing for chemotherapy or radiation therapy.
Several cryopreservation techniques have been developed since the first successful cryopreservation of human embryos was reported [3]. Currently, two basic techniques are used in human embryo cryopreservation: slow freezing and vitrification [4,5]. Both techniques can affect the developmental competence and function of embryos, as the embryos are exposed to various stressors during cryopreservation. Until now, the slow freezing technique has been the most widely used method for the cryopreservation of human embryos. The embryos are then loaded into a straw and slowly cooled to between -30Ōäā and -65Ōäā in a programmable cell freezer. The straw containing the embryos is plunged into liquid nitrogen (LN2) and stored until the embryos are used in a frozen-thawed ET cycle. Slow freezing is a time-consuming procedure and requires expensive equipment. Scientific interest in the application of vitrification to human embryo cryopreservation has increased due of its feasibility and clinical outcomes. During vitrification procedures, embryos are exposed to high concentrations of cryoprotectants and rapidly enter a glass-like state by exposure to rapid cooling rates (15,000 to 30,000Ōäā/min). Vitrification is a more time- and cost-efficient technique as compared to slow freezing given its use of rapid cooling rates, and does not require expensive instruments. However, several problems, such as the toxicity of cryoprotectants and the dangers of contamination remain [2,6].
Numerous studies have investigated the differences between slow freezing and vitrification. According to studies performed with human embryos, vitrification appears to result in higher survival and development to blastocyst rates [7-9]. Vitrification also results in higher implantation and pregnancy rates than slow freezing [10-12]. However, some animal studies have reported the potential harmful effects of vitrification on embryonic development [13,14]. In mice, [3H]2-deoxyglucose uptake and the implantation rates of blastocysts developed from vitrified 2-cell embryos were lower than those of fresh embryos or embryos frozen by slow freezing. Given this information, further studies are required to confirm the superiority of vitrification to slow freezing as a cryopreservation technique for human IVF and ET programs. The majority of studies comparing slow freezing and vitrification thus far have focused on survival rates, developmental rates after thawing and pregnancy rates. There are few studies investigating the effects of cryopreservation on cellular changes in embryos or the differences in cellular changes between embryos frozen by slow freezing or vitrification. Recently, differential expressions of 183 genes were reported in 8-cell stage mouse embryos frozen using the solid surface vitrification (SSV) technique [15]. In 2006, Boonkusol et al. [16] observed that the expression of stress-related genes increased at three hours after thawing in pronuclear embryos frozen by vitrification and that solid surface vitrification and in-straw vitrification expression of the genes showed differences. We hypothesized that slow freezing and vitrification might show differences in gene expression since different vitrification techniques showed differences in gene expression. Therefore, we examined the gene expression of mouse embryos frozen-thawed by slow freezing or vitrification and compared the level of gene expression for slow freezing and vitrification. In addition, the efficiencies of these two techniques were compared using survival, development to blastocyst after thawing, and cell number of blastocysts as indicators.
Female BDF1 mice (4-5 weeks old) were superovulated by intraperitoneal injection of 7.5 IU of pregnant mare serum gonadotropin (PMSG, Sigma, St Louis, MO, USA), followed 48 hours later by 7.5 IU of hCG (Sigma). The superovulated females were mated with a single male of the same strain (>10 weeks old). Mating was confirmed by the presence of a vaginal plug. Mated females were sacrificed by cervical dislocation and 8-cell embryos were collected by flushing the oviducts 62 hours after hCG injection. The embryos were washed in M16 medium (Sigma) and cultured in the same medium at 37Ōäā and 5% CO2 in air prior to freezing. Control embryos were continuously cultured to blastocysts without freezing. Development to blastocyst of the embryos was observed 115 hours after hCG injection in the control group.
Embryos were first placed in phosphate-buffered saline (PBS, Invitrogen, Grand Island, NY, USA) supplemented with 20% (v/v) synthetic serum substitute (SSS; Irvine Scientific, Santa Ana, CA, USA) at 37Ōäā for 5 minutes. The embryos were transferred to an equilibration medium consisting of 1.5 M propanediol (PROH, Sigma) in base medium and incubated at room temperature (24-25Ōäā) for 10 minutes. The embryos were then exposed to a freezing solution (1.5 M PROH and 0.1 M sucrose in base medium) for an additional 10 minutes. Following exposure to the freezing solution, embryos were loaded into 0.25-mL plastic straws and placed in a programmable freezer (Cryo Magic, Mirae Biotech, Seoul, Korea) for cooling. The embryos were cooled at a rate of -2Ōäā/min until they reached -7Ōäā. Seeding was carried out using forceps that had been placed in LN2 at -7Ōäā. Cooling was continued at a rate of -0.3Ōäā/min to -30Ōäā and then at a rate of -10Ōäā/min to -150Ōäā. The straw was inserted into LN2 for storage. In order to thaw the embryos, the straw was removed from the LN2, held at room temperature for 60 seconds, and then immersed in a water bath at 37Ōäā for one minute. The embryos were then expelled into a base medium containing 1M PROH, 0.2 M sucrose and suspended for five minutes at room temperature. For further rehydration and removal of cryoprotectants, the embryos were placed in a 0.5 M PROH, 0.2 M sucrose solution at room temperature for 5 minutes, a 0.2 M sucrose solution at 37Ōäā for 10 minutes, and base medium at 37Ōäā for 10 minutes. Finally, the embryos were washed five times in M16 medium and cultured to blastocysts in M16 medium at 37Ōäā, 5% CO2 in air. Development to blastocysts was observed 53 hours after thawing.
The embryos were washed in base medium consisting of PBS supplemented with 10% SSS and then suspended in an equilibration medium consisting of 7.5% ethylene glycol (EG, Sigma) and 7.5% dimethylsulphoxide (DMSO, Sigma) in a base medium at room temperature for 15 minutes. Following equilibration, the embryos were rinsed three times in small drops of vitrification solution consisting of 15% EG and 15% DMSO in base medium for 30 to 60 seconds. The embryos were then loaded into a modified pull and cut straw. The straw was made by pulling and cutting a 0.25-mL plastic straw. After loading the embryos, the straw was covered with another straw whose end was heat sealed and plunged into LN2.
For warming, the straws were removed from the LN2 and held in air for 10 seconds and then placed into 37Ōäā water for 1 minute. The contents of the straws were then expelled into a 37Ōäā 1 M sucrose solution for 1 minute. For further rehydration and removal of the cryoprotectants, the embryos were placed in a 0.5 M sucrose solution for 3 minutes and a base medium for 5 minutes at room temperature. The embryos were washed five times in M16 medium and cultured to blastocysts in M16 medium at 37Ōäā, 5% CO2 in air. Development to blastocysts was observed 53 hours after warming.
The nuclei of inner cell mass (ICM) and trophectoderm (TE) were stained differentially using the method described by Van der Elst et al. [17]. The blastocysts were preincubated for 30 minutesat room temperature with whole rabbit anti-mouse serum (Sigma) and then exposed to a 1:5 guinea pig complement solution (Sigma) containing propidium iodide (10 mg/mL) at 37Ōäā for 5 minutes. The blastocysts were fixed and counterstained in absolute ethanol containing bisbenzimide (20 mg/mL, Sigma). The blastocysts were washed in absolute ethanol overnight and mounted in glycerol on glass slides under light pressure. Cell counting was performed blindly by one investigator under ultraviolet illumination using florescent microscopy (BX61, Olympus, Tokyo, Japan) at 200├Ś magnification.
Total RNAs (tRNA) were extracted from 20 embryos of each group (control, slow freezing, and vitrification) using TRIzol (Invitrogen) according to the manufacturer's instructions and precipitated in the presence of 1 ┬ĄL of glycogen (20 mg/mL) (Sigma) as a nucleotide carrier. The RNA pellet was washed with 75% ethyl alcohol, air-dried, and eluted in 10 ┬ĄL of distilled water. For reverse transcription reactions, the isolated tRNA of each group was added to a reaction mixture containing 5 X M-MLV reverse transcriptase buffer (Promega, Madison, WI, USA), 40 U RNase inhibitor (RNasin, Promega), 10 mM dNTP mixture, 10 pM oligo-p(dT)15-primer, 200 U M-MLV reverse transcriptase, and RNase-free distilled water. The reaction mixtures were incubated at 37Ōäā for 1 hour followed by 5 minutes of incubation at 95Ōäā.
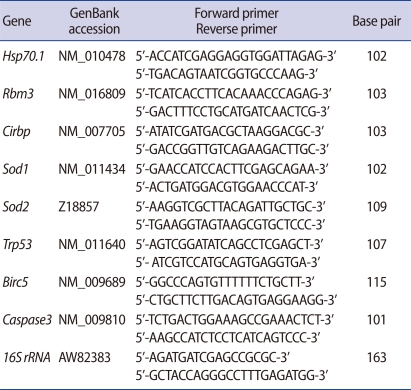
The expressions of eight genes were analyzed using real-time qPCR. In order to analyze gene expressions of fresh and thawed embryos frozen by slow freezing or vitrification, gene-specific primers were designed and used for RT-PCR. The analyzed genes, PCR primers, and sizes of the amplification products are summarized in Table 1. Real-time qPCR was performed with the DNA Engine Opticon2 system (Bio-Rad, Hercules, CA, USA) using a DyNAmo HS SYBR Green qPCR kit (Finnzymes, Espoo, Finland). The expression of each gene was normalized to the housekeeping 16 S ribosomal RNA within the log linear phase of the amplification curve using the comparative CT method. Melting curve analyses were performed for all real-time qPCR reactions to confirm the specificity and integrity of the PCR products.
All embryos were randomly distributed to experimental groups and three independent experiments were repeated. The survival rates, developmental rates, and cell numbers of blastocysts were analyzed with the Žć2 test. The results of the real-time qPCR were analyzed using an analysis of variance test. A p-values less than 0.05 were considered significant.
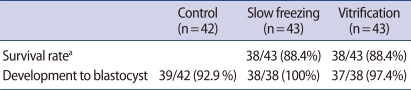
A total of 42 embryos were cultured to blastocysts as controls. Among them, 39 embryos developed into blastocysts (92.9%). Forty-three embryos were frozen by slow freezing and 43 were frozen by vitrification. The frozen embryos were all recovered after warming and morphological signs of damage were not observed in any of the surviving embryos. Thirty-eight of the 43 embryos (88.4%) frozen by slow freezing survived after thawing, all of which developed into blastocysts. Thirty-eight of the 43 embryos (88.4%) frozen by vitrification survived, of which 37 embryos (97.4%) developed into blastocysts. There were no statistically significant differences in the survival rates or development to blastocysts of embryos that underwent slow freezing or vitrification. Nor were there any statistically significant differences in development to blastocysts between the control embryos and the embryos underwent slow freezing or vitrification (Table 2).
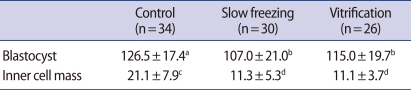
The cell numbers of the blastocysts and ICM were counted by differential staining in each experimental group (Table 3). In the control group (n=34), the cell numbers of blastocysts and ICM were 126.5┬▒17.4 and 21.1┬▒7.9, respectively. The cell numbers of blastocysts and ICM were 107.0┬▒21.0 and 11.3┬▒5.2 in the slow freezing group (n=30). The numbers of blastocyst cells and ICM cells of the vitrification group (n=26) were 115.0┬▒19.7 and 11.1┬▒3.7, respectively. The number of blastocyst cells of the control group were significantly higher (p<0.05) than those seen in the slow freezing and vitrification groups. The ICM cell numbers of the control group were significantly higher (p<0.001) than those of the experimental groups. However, the numbers of blastocyst and ICM cells were not significantly different in the slow freezing and vitrification groups.
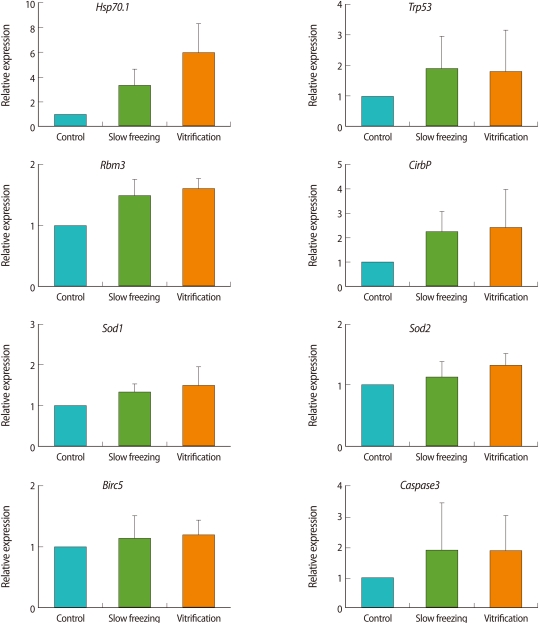
The expressions of the eight genes were analyzed in fresh and frozen-thawed embryos using real-time qPCR. The expressions of the genes were normalized to the expression of the housekeeping gene, 16 S rRNA (Figure 1). Compared to the control group, the expression of Hsp70.1 was increased threefold in the slow freezing group and about sixfold in the vitrification group. The expression of CirbP was two times higher in the frozen-thawed embryos than in the control embryos. However, there were no statistically significant differences in the among the groups' expressions of Hsp70.1 and CirbP. The expressions of Trp53 and Caspase3 were higher in the frozen-thawed embryos than in the fresh embryos, but the difference was not significant. Nor did the expressions of the slow freezing group and vitrification group differ. The expressions of Rbm3, Birc5, Sod1, and Sod2 did not differ among the control groups, slow freezing group, and vitrification group.
Several studies comparing slow freezing and vitrification have been performed using animal and human embryos. Most of these studies have focused on survival and development after thawing [8,9]. A few studies have examined differences in metabolism and pregnancy outcomes [7,10,11,13]. However, these studies are insufficient for understanding the cellular changes caused by cryopreservation. To the best of our knowledge, this is the first study comparing the effect of two cryopreservation techniques, slow freezing and vitrification, on gene expression in mouse embryos as a part of the effort to understand cellular changes in embryos that are caused by cryopreservation. In addition, we were able to compare the efficiencies of the two techniques using survival, development to blastocyst after thawing, and number of blastocyst cells as indicators.
In the present study, the survival and developmental rates after thawing of the slow freezing and vitrification embryos did not differ significantly. The developments of frozen-thawed embryos and fresh embryos were similar (Table 2). In contrast to this study, several studies have reported that the developmental rates of cryopreserved embryos were different from those of fresh embryos [18,19]. In particular, Uechi et al. [13] reported that the development rate of vitrified 2-cell embryos was lower than embryos frozen by slow freezing. Several factors may explain the differences in developmental rates between the present study and previous studies. For example, the developmental stage at which the mouse embryos were cryopreserved, the constitution of cryoprotectants, and the containers used to load embryos were all variables tailored specifically to our study. In contrast with other studies, which used early stage (2-cell) embryos for cryopreservation, our study used late stage embryos (8-cell). Cryopreservation of late stage embryos, such as 8-cell embryos or morulae, resulted in better development to blastocysts after thawing than did that of early stage embryos [20]. Concentrations of cryoprotectants in the vitrification solution were higher in previous studies than in the present study. Cryoprotectants are essential for cryopreservation of embryos but are also toxic [2]. The higher concentrations of cryoprotectants in previous studies may have had a detrimental effects on embryonic development. The cooling rate is the most important parameter, and increased cooling rates have been shown to result in higher rates of successful vitrification [21-23]. Embryos were loaded into 0.25-mL plastic straws in previous studies. However, we used a modified pull and cut straw to achieve higher cooling rates and to minimize the volume of the vitrification solution in which embryos were loaded. A higher cooling rate may have been achieved by using the modified pull and cut straw. These differences between previous studies and our study may have resulted in the improved developmental rate of thawed embryos frozen by vitrification that was seen in this study.
It has been reported that the cell numbers of blastocysts decrease after the cryopreservation of mouse oocytes or embryos [13,17]. Similar results were obtained in this study. The cell numbers of blastocysts developed from frozen-thawed embryos were significantly reduced compared with blastocysts developed from fresh embryos. However, the cell numbers of blastocysts of the two experimental groups did not differ from each other (Table 3). Van der Elst et al. [17] reported that delayed cleavage and disturbed allocation of cells to ICM and TE may be the reason for the decrease in cell number after cryopreservation. Like Van der Elst et al. [17], we also observed a decrease in the ICM-to-TE ratio, along with a reduced cell number of blastocysts after cryopreservation, in blastocysts developed from frozen-thawed 8-cell embryos (data not shown). If the delay development were the only cause of the decreased cell number, the ICM-to-TE ratio would not differ between the control blastocysts and blastocyts developed from frozen-thawed embryos. However, the ratio was lower in blastocyts developed from frozen-thawed embryos, and it seems that allocation of cells to ICM and TE is disturbed in frozen-thawed embryos. It also appears that this disturbed allocation of cells caused the decrease in the number of ICM cells. Embryos with reduced cell numbers are able to give rise to fetuses [24-26]. However, the decreased cell number of embryos, especially ICM cell numbers, may cause preclinical abortions or blighted ova [27].
There is a lack of information on cellular changes caused by cryopreservation. In a study investigating the effects of several vitrification techniques on gene expression in embryos thawed after cryopreservation, Boonkusol et al. [16] reported that gene expression in embryos can be affected by cryopreservation techniques. According to the study, in-straw treated pronuclear stage mouse embryos showed higher activity of stress-related genes than SSV-treated and fresh embryos 3 hours post-warming. Additionally, there were no changes in gene expression in the 8-cell stage mouse embryos. Similar to those results, gene expression of the fresh embryos and frozen-thawed embryos did not differ in this study. Nor was there a significant difference in gene expression between slow freezing and vitrification. Boonkusol et al. [16] have reported that stress-related genes in pronuclear embryos showed different expression levels with solid surface vitrification than with in-straw vitrification. Therefore, we analyzed the stress-related genes that Boonkusol et al. [16] have analyzed. We also analyzed the expression of caspase-3 and Birc5 genes to evaluate the effect of cryopreservation on the apoptosis of embryos. Although the expressions of Hsp70.1 and CirbP were higher in the frozen-thawed embryos, the expression of the eight genes analyzed did not differ among the groups. However, only eight genes were investigated in this study. It is possible that differences in gene expression between the two techniques could be detected by investigating the expression of additional genes; this requires further study to better understand the changes in embryo cells caused by cryopreservation.
We have shown that survival, developmental potential, cell number of blastocysts, and gene expression pattern after thawing did not differ by using slow freezing and vitrification methods for 8-cell stage mouse embryos. However, compared with fresh embryos, the numbers of blastocyst cells were significantly lower and the expressions of several stress-related genes were significantly higher after freezing and thawing. Cellular changes, such as elevated gene expression after cryopreservation, could cause delayed development and disturbed allocation of cells resulting in a decrease in the numbers of blastocyst cells. According to studies thus far, vitrification appears to be superior to slow freezing in terms of survival and development after thawing in human IVF and ET programs. Vitrification has the potential to become more attractive than slow freezing in ART laboratories. However, the effects of slow freezing and vitrification on pregnancy outcomes in human IVF and ET programs are still a matter of controversy. Therefore, further studies to confirm the advantages of vitrification and refine the technique are needed.
References
1. Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet 1978;2:366PMID: 79723.

2. Liebermann J, Dietl J, Vanderzwalmen P, Tucker MJ. Recent developments in human oocyte, embryo and blastocyst vitrification: where are we now? Reprod Biomed Online 2003;7:623-633.PMID: 14748959.


3. Trounson A, Mohr L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature 1983;305:707-709.PMID: 6633637.


4. Cohen J, DeVane GW, Elsner CW, Fehilly CB, Kort HI, Massey JB, et al. Cryopreservation of zygotes and early cleaved human embryos. Fertil Steril 1988;49:283-289.PMID: 3338585.


5. Rama Raju GA, Haranath GB, Krishna KM, Prakash GJ, Madan K. Vitrification of human 8-cell embryos, a modified protocol for better pregnancy rates. Reprod Biomed Online 2005;11:434-437.PMID: 16274602.


6. Bielanski A, Nadin-Davis S, Sapp T, Lutze-Wallace C. Viral contamination of embryos cryopreserved in liquid nitrogen. Cryobiology 2000;40:110-116.PMID: 10788310.


7. Balaban B, Urman B, Ata B, Isiklar A, Larman MG, Hamilton R, et al. A randomized controlled study of human Day 3 embryo cryopreservation by slow freezing or vitrification: vitrification is associated with higher survival, metabolism and blastocyst formation. Hum Reprod 2008;23:1976-1982.PMID: 18544577.


8. Loutradi KE, Kolibianakis EM, Venetis CA, Papanikolaou EG, Pados G, Bontis I, et al. Cryopreservation of human embryos by vitrification or slow freezing: a systematic review and meta-analysis. Fertil Steril 2008;90:186-193.PMID: 17980870.


9. Kolibianakis EM, Venetis CA, Tarlatzis BC. Cryopreservation of human embryos by vitrification or slow freezing: which one is better? Curr Opin Obstet Gynecol 2009;21:270-274.PMID: 19276976.


10. Rezazadeh Valojerdi M, Eftekhari-Yazdi P, Karimian L, Hassani F, Movaghar B. Vitrification versus slow freezing gives excellent survival, post warming embryo morphology and pregnancy outcomes for human cleaved embryos. J Assist Reprod Genet 2009;26:347-354.PMID: 19513822.



11. Lin TK, Su JT, Lee FK, Lin YR, Lo HC. Cryotop vitrification as compared to conventional slow freezing for human embryos at the cleavage stage: survival and outcomes. Taiwan J Obstet Gynecol 2010;49:272-278.PMID: 21056310.


12. AbdelHafez FF, Desai N, Abou-Setta AM, Falcone T, Goldfarb J. Slow freezing, vitrification and ultra-rapid freezing of human embryos: a systematic review and meta-analysis. Reprod Biomed Online 2010;20:209-222.PMID: 20113959.


13. Uechi H, Tsutsumi O, Morita Y, Takai Y, Taketani Y. Comparison of the effects of controlled-rate cryopreservation and vitrification on 2-cell mouse embryos and their subsequent development. Hum Reprod 1999;14:2827-2832.PMID: 10548631.


14. Naik BR, Rao BS, Vagdevi R, Gnanprakash M, Amarnath D, Rao VH. Conventional slow freezing, vitrification and open pulled straw (OPS) vitrification of rabbit embryos. Anim Reprod Sci 2005;86:329-338.PMID: 15766810.


15. Mamo S, Bodo S, Kobolak J, Polgar Z, Tolgyesi G, Dinnyes A. Gene expression profiles of vitrified in vivo derived 8-cell stage mouse embryos detected by high density oligonucleotide microarrays. Mol Reprod Dev 2006;73:1380-1392.PMID: 16897732.


16. Boonkusol D, Gal AB, Bodo S, Gorhony B, Kitiyanant Y, Dinnyes A. Gene expression profiles and in vitro development following vitrification of pronuclear and 8-cell stage mouse embryos. Mol Reprod Dev 2006;73:700-708.PMID: 16541460.


17. Van der Elst J, Amerijckx Y, Van Steirteghem A. Ultra-rapid freezing of mouse oocytes lowers the cell number in the inner cell mass of 5 day old in-vitro cultured blastocysts. Hum Reprod 1998;13:1595-1599.PMID: 9688398.


18. Selick CE, Hofmann GE, Albano C, Horowitz GM, Copperman AB, Garrisi GJ, et al. Embryo quality and pregnancy potential of fresh compared with frozen embryos--is freezing detrimental to high quality embryos? Hum Reprod 1995;10:392-395.PMID: 7769069.


19. Uechi H, Tsutsumi O, Morita Y, Taketani Y. Cryopreservation of mouse embryos affects later embryonic development possibly through reduced expression of the glucose transporter GLUT1. Mol Reprod Dev 1997;48:496-500.PMID: 9364444.


20. Miyake T, Kasai M, Zhu SE, Sakurai T, Machida T. Vitrification of mouse oocytes and embryos at various stages of development in an ethylene glycol-based solution by a simple method. Theriogenology 1993;40:121-134.PMID: 16727299.


21. Arav A, Zeron Y, Ocheretny A. A new device and method for vitrification increases the cooling rate and allows successful cryopreservation of bovine oocytes. Theriogenology 2000;53:248-249.
22. Hochi S, Akiyama M, Minagawa G, Kimura K, Hanada A. Effects of cooling and warming rates during vitrification on fertilization of in vitro-matured bovine oocytes. Cryobiology 2001;42:69-73.PMID: 11336491.


23. Isachenko V, Alabart JL, Nawroth F, Isachenko E, Vajta G, Folch J. The open pulled straw vitrification of ovine GV-oocytes: positive effect of rapid cooling or rapid thawing or both? Cryo Letters 2001;22:157-162.PMID: 11788855.

24. Iwasaki S, Yoshiba N, Ushijima H, Watanabe S, Nakahara T. Morphology and proportion of inner cell mass of bovine blastocysts fertilized in vitro and in vivo. J Reprod Fertil 1990;90:279-284.PMID: 2231548.


25. Loskutoff NM, Johnson WH, Betteridge KJ. The developmental competence of bovine embryos with reduced cell numbers. Theriogenology 1993;39:95-107.

26. Tao T, Reichelt B, Niemann H. Ratio of inner cell mass and trophoblastic cells in demi- and intact pig embryos. J Reprod Fertil 1995;104:251-258.PMID: 7473416.


27. Edwards RG. Causes of early embryonic loss in human pregnancy. Hum Reprod 1986;1:185-198.PMID: 3305553.


Figure┬Ā1
Relative expressions of the eight genes in thawed 8-cell mouse embryos frozen by slow freezing or vitrification. There was no significant difference in gene expressions among the groups. Relative expression levels are expressed as mean┬▒SE.
Table┬Ā1
Genes, primers, and sizes of amplification products (bp) for quantification of gene expression by real-time quantitative polymerase chain reaction