|
 |
- Search
| Clin Exp Reprod Med > Volume 43(3); 2016 > Article |
Abstract
Objective
This study was conducted to compare the effects of static culture, dynamic culture, and the combination of dynamic culture with specialized surfaces involving co-culture on human embryonic development. Embryos cultured using conventional static culture (SC) techniques served as a control group. We compared dynamic culture using micro-vibration culture (MVC) and micro-vibration with co-culture (MCoC), in which autologous cumulus cells were used as a specialized surface.
Methods
We conducted a chart review of patients who were treated between January 2011 and November 2014 in order to compare embryonic development rates and pregnancy rates among the groups. Zygotes were cultured in micro-droplets, and embryos were subsequently selected for transfer. Some surplus embryos were cryopreserved, and the others were cultured for blastocyst development. A micro-vibrator was set at the frequency of 42 Hz for duration of 5 seconds per 60 minutes to facilitate embryo development.
Results
No significant differences among the groups were present in patient's characteristics. However, the clinical pregnancy rates were significantly higher in the MVC group and the MCoC group than in the SC group. No significant differences were found in the blastocyst development rate between the SC group and the MVC group, but the blastocyst development rate in the MCoC group was significantly higher than in the SC and MVC groups.
The embryo culture techniques used in assisted reproductive technology (ART) have undergone a continual process of improvement. One common method of improving embryo culture techniques is to develop a medium for embryos in vitro, and many researchers have developed various media with this goal in mind. Several types of culture media have proven compatible with embryo cultures and are commercially available from a broad range of manufacturers. Another common strategy is developing culture platforms. Recent culture platform studies have attempted to overcome the problems and limitations of existing culture systems [1]. For example, modifying the physical environment by altering embryo spacing was shown to maximize autocrine/paracrine effects, secreted factors, and the extent of group culture effects. This culture platform is referred to as the new static culture platform and includes a well-of-the-well system, micro-well culture, sub-micro-drop culture, and GPS dish culture (the GPS dish for embryos is designed to ensure effective observation and safe handling of oocytes and embryos) [2,3,4,5,6,7]. The dynamic culture platform aims to provide an environment similar to that of the body during germ cell development in vitro. Before implantation, the oocytes and zygotes within the oviduct and uterus are constantly moving and stimulated by in vivo mechanical forces, such as the beating of the cilia, pulsatile muscle contraction of the uterus and oviduct, and sperm motility [8,9,10,11]. This platform uses various external stimuli to support embryo movement and stimulation, incorporating micro-funnels, tilting, micro-vibration, and shaking machines [12,13,14,15,16]. Specialized surface platforms have also been used to study embryo cultures. Embryo development was shown to be improved by physiological culture compared with culture on plastic surfaces. Embryos in the reproductive tract are surrounded by a range of polyhydroxylated compounds, macromolecules, and extracellular matrix components [17,18]. Various polyhydroxylated compounds and functional groups characteristic of macromolecules, such as hydroxyl, amine, and carboxyl groups, can affect embryo metabolism by bonding or adhering to water and nutrient molecules [19]. Therefore, in embryo culture research, many attempts have been made to achieve physiologically realistic surfaces for embryo culture [20,21,22]. Three types of advanced culture platforms have been studied. Each culture platform has its own specific advantages, and in our study, we attempted to combine the embryo culture platforms to leverage these advantages.
We previously found that the total pregnancy and implantation rates were significantly higher in embryos from poor responders subjected to micro-vibration culture than in embryos subjected to static culture [23]. We also reported that including a cumulus cell monolayer improved embryo quality and the blastocyst development rate [24]. Cumulus cell co-culture has been shown to improve the maturation rate of immature oocytes as well as the blastocyst development rate [25,26,27]. Co-culture also enables surface modifications and exposure to the extracellular matrix and other compounds that may influence embryo development, and co-cultures using cumulus cells have therefore been recognized as a type of specialized surface platform [28]. With this in mind, it would be desirable to develop a new embryo culture method based on the long-term accumulation of co-culturing experience and studies of dynamic culture platforms incorporating micro-vibration.
Poor responders tend to be of advanced age and to have a history of previous ovarian surgery or pelvic adhesions, which can contribute to reduced oocyte and embryo quality in comparison to normal responders [29,30]. Various approaches have been developed to improve the pregnancy rate in poor responders, but a satisfactory solution has not yet been identified [31]. The aim of ART is to culture better-quality embryos that can lead to pregnancy.
The purpose of this study was to improve embryo development and clinical outcomes in poor responders using static and dynamic culture platforms and dynamic culture with a specialized surface. This study is the first to combine dynamic culture (incorporating micro-vibrations) and a specialized surface platform (a cumulus cell monolayer) to improve human embryonic development.
This prospective randomized study was performed at Maria Fertility Hospital. A chart review of poor responders from January 2011 to November 2014 was conducted according to the policy of the Maria Fertility Hospital Institutional Review Board (approval number 2015-002).
In our hospital, protocols for poor responders are routinely implemented in order to maximize the number of eggs. Some patients were administered a gonadotropin-releasing hormone (GnRH) agonist on the second or third day of the menstrual cycle for 3 days, followed by a GnRH antagonist to prevent premature ovulation. Some patients were administered only a low dose of the GnRH agonist, whereas others were administered the GnRH agonist during the luteal phase, with discontinuation at the beginning of the menstrual cycle. Additionally, other patients followed a modified natural cycle.
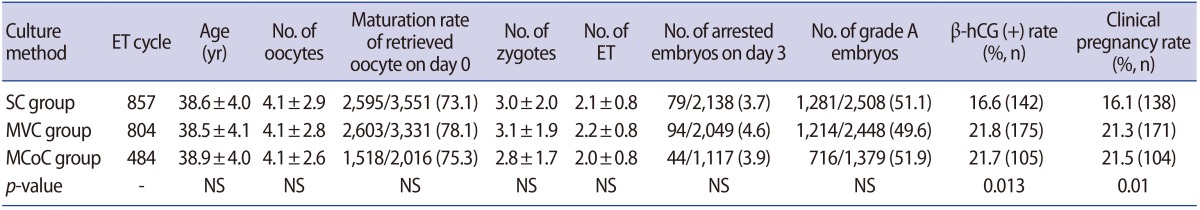
Poor responders were divided into a static culture (SC) group, a micro-vibration culture (MVC) group, and a micro-vibration with co-culture (MCoC) group according to the culture method used. The SC, MVC, and MCoC groups included 857, 804, and 484 cycles, respectively. Group differences in maternal age, number of oocytes, number of embryos, and number of transferred embryos were analysed. Pregnancy was assessed by serum human chorionic gonadotropin (hCG) levels 14 days after progesterone administration. Implantation was confirmed by the presence of a gestational sac.
Oocyte retrieval was performed through transvaginal ultrasound-guided aspiration after 36 hours of administering 10,000 IU of hCG (IVF-C, LG Life Science, Daejeon, Korea). The retrieved oocytes were washed in fresh medium (Sydney IVF Fertilization Medium-FM, Cook, Brisbane, Australia) and then cultured in the same medium (Sydney IVF Fertilization Medium-FM, Cook) until in vitro fertilization. In vitro fertilization was induced using conventional insemination or intracytoplasmic sperm injection. Fertilization was assessed 15 to 18 hours after insemination based on the presence of two pronuclei. The zygotes were washed and cultured in groups of less than five in a 50-┬ĄL micro-droplet (Sydney IVF Cleavage Medium-CM, Cook) for 48 hours. Embryos were subsequently selected for transfer. Some surplus embryos were cultured in a 50-┬ĄL micro-droplet (Sydney IVF Blastocyst Medium-BM, Cook) for blastocyst development. All embryo cultures were maintained in a CO2:O2:N2 (6%:5%:89%) environment.
A micro-vibrator (NSSB-300, Nepa Gene, Ichikawa, Japan) was set at a frequency of 42 Hz for a duration of 5 seconds per 60 minutes in order to optimally facilitate human embryonic development in MVC, based on previous reports [24].
Autologous cumulus cells were prepared for MCoC in 5-┬ĄL micro-droplets in a two-well culture dish (3653, BD Falcon, Durham, NC, USA) under mineral oil (Ovoil, Vitrolife, Kungsbacka, Sweden). Cultures were prepared by seeding single cells, followed by excising them from a clear corona radiata of healthy cumuli and digesting them with hyaluronidase (MRC#Hyase, Biosupply, Seoul, Korea). The cumulus cell-seeded two-well culture dish was washed with fresh medium (Sydney IVF Cleavage Medium-CM, Cook) to remove any unattached cumulus cells [32]. The cumulus cell monolayer dish was washed again with fresh co-culture medium after the assessment of fertilization.
Embryos were selected for transfer on day 3. The number of embryos for transfer was limited to two, although some poor responders received three embryos, depending on their clinical history (e.g., repeated failures or old age). Generally, the cell division rate in poor responders is relatively slow due to poor oocyte quality and quantity. Therefore, based on the report published by Ziebe et al. [33], the embryos were divided into three grades: A, B, and C, corresponding to cell stages 6ŌĆō8 (<10% fragmentation), 4ŌĆō5 (fragmentation between 10% and 30%), and <4 (30% fragmentation), respectively. After transfer, some of the surplus embryos were cultured to assess blastocyst development under the same conditions. Others were cryopreserved at the cleavage stage, and the remaining embryos were discarded. The blastocysts were evaluated on days 5, 6, and 7 according to Gardner's grading methods and cryopreserved or discarded based on their quality [34].
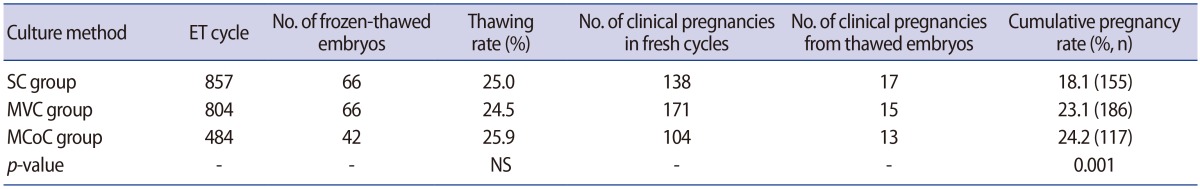
The clinical pregnancy rates of the frozen-thawed embryos in the SC, MVC, and MCoC groups were also analysed. Based on the pregnancy rates obtained using fresh cycles and frozen-thawed cycles in each group, the cumulative pregnancy rate was also analysed.
The SC, MVC, and MCoC groups contained 857, 804 and 484 cycles, respectively. No significant differences in age, number of oocytes, oocyte maturity on day 0, zygote number, or embryo transfer number were observed between the groups (Table 1). The numbers of grade A embryos and arrested embryos were similar among the groups on day 3. Consistent with our previous study, the influence of MVC on the cleavage stage of embryo development was found to be small. However, the hormonal and clinical pregnancy rates were significantly higher in the MVC and MCoC groups (Table 1). The lack of significant differences in pregnancy rates between the MVC and MCoC groups indicated that co-culture had no significant effect on the pregnancy rate in poor responders.
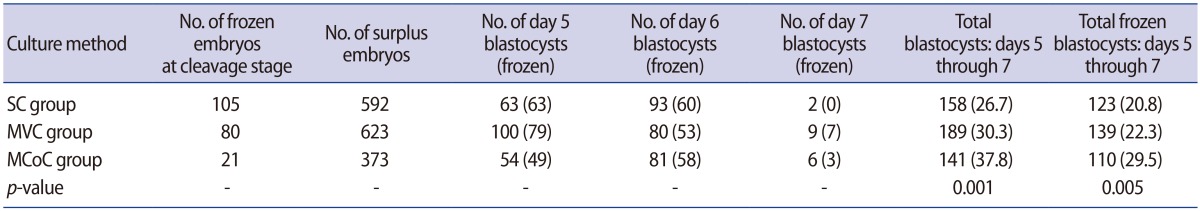
With the exception of the embryos that were cryopreserved at the cleavage stage, the SC, MVC and MCoC groups contained a surplus of 592, 623, and 373 embryos, respectively. From day 5 until day 7, the number of developed blastocysts in the SC, MVC and MCoC groups totalled 158, 189, and 141, respectively (Table 2). The blastocyst development rate was higher in the MVC group than in the SC group, but this difference was not statistically significant. However, the blastocyst development rate in the MCoC group was significantly higher than in the other groups. The proportion of frozen blastocysts was significantly higher in the MCoC group than in the other groups (Table 2). MVC exhibited a more positive effect on blastocyst development in human embryos compared with the effect obtained with SC, but this difference was not statistically significant. MCoC had a greater positive effect on the blastocyst development rate in human embryos than either SC or MVC.
The clinical pregnancy rates of the frozen-thawed cycles were examined for each group, and the thawing rates of frozen-thawed cycles were found to be similar among the groups (Table 3). The cumulative pregnancy rate of the MCoC group exceeded those of the SC and MVC groups. Since the blastocyst freezing rate of the MCoC group was significantly higher than was observed in the SC and MVC groups, differences between the cumulative pregnancy rate and the pregnancy rate of fresh cycles would have been expected to become increasingly larger as the thawing rates increase.
Two major pathways of improving embryo culture include performing culture platform studies, in which embryo culture conditions are changed, and developing culture media capable of effectively culturing embryos in vitro. Many types of culture media are commercially available and have been studied extensively. Culture platforms are currently classified as new static, dynamic, and specialized surface platforms. Each type of culture platform offers specific advantages [35], and the benefits of each type should be combined to gain insight into the physiological environment of embryonic culture in vitro and how to develop improved culture platforms. We created a novel combination of dynamic culture and specialized surface culture platforms to assess the impact of such a combination on human embryonic development.
Due to the better quality of oocytes and embryos in normal responders, human embryonic development and clinical results have been found to be significantly improved by MVC [36]. In poor responders, large doses of stimulant medications, sub-optimal oocyte quantity, poor embryo quality, old age, and defective endometria have been associated with poor prognoses. We have previously reported differences in embryo quality between SC and MVC at the cleavage stage and observed significantly increased pregnancy and implantation rates when human embryos were subjected to MVC [24]. In this study, the quality of the cleavage-stage embryos on day 3 was similar in the SC, MVC, and MCoC groups. The co-culture method did not visibly affect cleavage-stage embryo quality, and the pregnancy and implantation rates were found to be significantly greater in the MVC and MCoC groups. Therefore, a cumulative positive effect on embryo development in poor responders can significantly influence clinical outcomes, and MVC was found to weakly improve the cleavage-stage embryo development rate. Micro-vibration mimics the in vivo mechanical forces in the oviduct fluid. The dynamic environments of these cultures are believed to cause cumulative positive effects on embryonic development by accelerating communication between cells or between cells and embryos through autocrine/paracrine or nutrient contact [37].
This study identified differences in the blastocyst development rate among the SC, MVC and MCoC groups. This rate was higher in the MVC group than in the SC group, but not to a statistically significant extent. However, the blastocyst development rate was significantly higher in the MCoC group than in the SC and MVC groups. Therefore, MCoC has the effect of increasing the blastocyst development rate to a greater extent than MVC or SC. Specialized surfaces offer intriguing means of improving current in vitro embryo culture techniques. In particular, cumulus cell co-culture may be a valuable tool for identifying interactions between embryos and reproductive tract cells with specific roles in embryonic development [26]. The MVC and co-culture methods are likely to cause synergetic effects on blastocyst development. Based on the positive results obtained using previously described dynamic culture methods, the cumulus cell co-culture environment provides embryos with a more physiologically realistic surface than plastic dishes. The culture condition used in this study involved a 50-┬ĄL micro-droplet of defined media with a partial monolayer of cumulus cells. Since the commercially available medium (Sydney IVF medium) was not designed for cumulus cell co-culture, we considered a partial monolayer of cumulus cells more appropriate than an entire monolayer within a droplet. Too many cumulus cells in a specific medium containing limited nutrients may have a negative effect on embryonic development. Although a partial monolayer of less than 50 cumulus cells with human embryos may not be the most preferable culture environment, it appears to be sufficient to exert a synergetic effect together with micro-vibration stimulation. More research is needed to determine the optimal number of cumulus cells for a specified volume of standard, commercially available medium. The combination of the various secretomes of cumulus cells and embryos, macromolecules on the surface cumulus cells, water-binding macromolecular functional groups, and increased cell-to-embryo communication resulting from media circulation in dynamic culture mimics physiological environments more closely than standard culture conditions. The blastocyst development rate of the MCoC group (37.8%) was nearly equal to that observed in surplus embryos from normal responders (38.0%) over the same period. We cultured the surplus embryos of normal responders using commercially available media (Cook or MRC) without adding micro-vibration stimuli. As the number of cryopreserved embryos increases, the cumulative pregnancy rate also increases. The cumulative pregnancy rate of the MCoC group was higher than was observed in the SC and MVC groups. The synergetic effects of MCoC not only affected the blastocyst development rate, but also contributed to improvements in the cumulative pregnancy rate.
Despite the many advantages of dynamic and specialized surface culture techniques, dynamic culture equipment and specialized surface materials have limited applicability in clinical settings. The micro-fluidic micro-funnel [38] has been evaluated in the most advanced dynamic culture platforms. These methods are structurally complex, and practical products implementing these methods have yet to be introduced. Although they are structurally simple, tilting embryo culture systems [17] are not yet commercially available. Only micro-vibration devices are generally commercially available for clinics. Thus, this system needs to be developed in combination with the closely related dynamic culture equipment available in clinical settings. Specialized surface platform materials have not undergone substantial recent improvement [1,18], and currently, cumulus cells are the easiest to use. The development of other types of material to create specialized surfaces is needed. Further research into, for example, various combinations of culture platforms, is needed to create the optimal human embryonic culture system.
References
1. Swain JE. Decisions for the IVF laboratory: comparative analysis of embryo culture incubators. Reprod Biomed Online 2014;28:535-547.PMID: 24656561.


2. Ali J. Continuous ultra micro-drop culture yields higher pregnancy and implantation rates than either large-drop culture or fresh-medium replacement. Clin Embryol (Online) 2004;7:1-23.
3. Gopichandran N, Leese HJ. The effect of paracrine/autocrine interactions on the in vitro culture of bovine preimplantation embryos. Reproduction 2006;131:269-277.PMID: 16452720.


4. Melin J, Lee A, Foygel K, Leong DE, Quake SR, Yao MW. In vitro embryo culture in defined, sub-microliter volumes. Dev Dyn 2009;238:950-955.PMID: 19301395.



5. Rieger D, Schimmel T, Cohen J, Cecchi M. Comparison of GPS and standard dishes for embryo culture: set-up and observation times, and embryo development In: 14th World Congress on In Vitro Fertilization; 2007 Sep 15-19; Montreal, Canada.
6. Vajta G, Peura TT, Holm P, Paldi A, Greve T, Trounson AO, et al. New method for culture of zona-included or zona-free embryos: the Well of the Well (WOW) system. Mol Reprod Dev 2000;55:256-264.PMID: 10657044.


7. Vajta G, Korosi T, Du Y, Nakata K, Ieda S, Kuwayama M, et al. The Well-of-the-Well system: an efficient approach to improve embryo development. Reprod Biomed Online 2008;17:73-81.PMID: 18616894.


8. Anand S, Guha SK. Mechanics of transport of the ovum in the oviduct. Med Biol Eng Comput 1978;16:256-261.PMID: 308127.


10. Lyons RA, Saridogan E, Djahanbakhch O. The reproductive significance of human Fallopian tube cilia. Hum Reprod Update 2006;12:363-372.PMID: 16565155.



11. Zervomanolakis I, Ott HW, Hadziomerovic D, Mattle V, Seeber BE, Virgolini I, et al. Physiology of upward transport in the human female genital tract. Ann N Y Acad Sci 2007;1101:1-20.PMID: 17416925.


12. Raty S, Walters EM, Davis J, Zeringue H, Beebe DJ, Rodriguez-Zas SL, et al. Embryonic development in the mouse is enhanced via microchannel culture. Lab Chip 2004;4:186-190.PMID: 15159776.


13. Cabrera LM, Heo YS, Ding J, Takayama S, Smith GD. Improved blastocyst development with microfluidics and Braille pin actuator enabled dynamic culture. Fertil Steril 2006;86:S43.

14. Mizobe Y, Yoshida M, Miyoshi K. Enhancement of cytoplasmic maturation of in vitro-matured pig oocytes by mechanical vibration. J Reprod Dev 2010;56:285-290.PMID: 20103989.


15. Isachenko E, Maettner R, Isachenko V, Roth S, Kreienberg R, Sterzik K. Mechanical agitation during the in vitro culture of human pre-implantation embryos drastically increases the pregnancy rate. Clin Lab 2010;56:569-576.PMID: 21141442.

16. Matsuura K, Hayashi N, Kuroda Y, Takiue C, Hirata R, Takenami M, et al. Improved development of mouse and human embryos using a tilting embryo culture system. Reprod Biomed Online 2010;20:358-364.PMID: 20093091.


17. Hunter SK, Scott JR, Hull D, Urry RL. The gamete and embryo compatibility of various synthetic polymers. Fertil Steril 1988;50:110-116.PMID: 3384103.


18. Hunter RH. Modulation of gamete and embryonic microenvironments by oviduct glycoproteins. Mol Reprod Dev 1994;39:176-181.PMID: 7826619.


19. Franks F. Solute-water interactions: do polyhydroxy compounds after the properties of water? Cryobiology 1983;20:335-345.PMID: 6884075.


20. Gardner DK, Rodriegez-Martinez H, Lane M. Fetal development after transfer is increased by replacing protein with the glycosaminoglycan hyaluronan for mouse embryo culture and transfer. Hum Reprod 1999;14:2575-2580.PMID: 10527990.


22. Villa-Diaz LG, Nandivada H, Ding J, Nogueira-de-Souza NC, Krebsbach PH, O'Shea KS, et al. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat Biotechnol 2010;28:581-583.PMID: 20512122.




23. Hur YS, Park JH, Ryu EK, Park SJ, Lee JH, Lee SH, et al. Effect of micro-vibration culture system on embryo development. J Assist Reprod Genet 2013;30:835-841.PMID: 23657828.



24. Lee SW, Yoon SH, Yoon HG, Cho HJ, Heo YS, Yoon HJ, et al. The studies on the development of human blastocyst embryos in IVF-ET Program. II: the development of human blastocyst embryos by co-culture with cumulus cells. Korean J Fertil Steril 1998;25:35-42.
25. Goto K, Kajihara Y, Kosaka S, Koba M, Nakanishi Y, Ogawa K. Pregnancies after co-culture of cumulus cells with bovine embryos derived from in-vitro fertilization of in-vitro matured follicular oocytes. J Reprod Fertil 1988;83:753-758.PMID: 3411565.


26. Goovaerts IG, Leroy JL, Van Soom A, De Clercq JB, Andries S, Bols PE. Effect of cumulus cell coculture and oxygen tension on the in vitro developmental competence of bovine zygotes cultured singly. Theriogenology 2009;71:729-738.PMID: 18962875.


27. Benkhalifa M, Demirol A, Sari T, Balashova E, Tsouroupaki M, Giakoumakis Y, et al. Autologous embryo-cumulus cells co-culture and blastocyst transfer in repeated implantation failures: a collaborative prospective randomized study. Zygote 2012;20:173-180.PMID: 21473794.


28. Swain JE, Smith GD. Advances in embryo culture platforms: novel approaches to improve preimplantation embryo development through modifications of the microenvironment. Hum Reprod Update 2011;17:541-557.PMID: 21454356.


29. Jenkins JM, Davies DW, Devonport H, Anthony FW, Gadd SC, Watson RH, et al. Comparison of 'poor' responders with 'good' responders using a standard buserelin/human menopausal gonadotrophin regime for in-vitro fertilization. Hum Reprod 1991;6:918-921.PMID: 1761658.



30. Keay SD, Liversedge NH, Mathur RS, Jenkins JM. Assisted conception following poor ovarian response to gonadotrophin stimulation. Br J Obstet Gynaecol 1997;104:521-527.PMID: 9166190.


31. Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L, et al. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod 2011;26:1616-1624.PMID: 21505041.


32. Kang SM, Lee SW, Jeong HJ, Yoon SH, Koh MW, Lim JH, et al. Clinical outcomes of elective single morula embryo transfer versus elective single blastocyst embryo transfer in IVF-ET. J Assist Reprod Genet 2012;29:423-428.PMID: 22382643.



33. Ziebe S, Petersen K, Lindenberg S, Andersen AG, Gabrielsen A, Andersen AN. Embryo morphology or cleavage stage: how to select the best embryos for transfer after in-vitro fertilization. Hum Reprod 1997;12:1545-1549.PMID: 9262293.



34. Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril 2000;73:1155-1158.PMID: 10856474.


35. Hickman DL, Beebe DJ, Rodriguez-Zas SL, Wheeler MB. Comparison of static and dynamic medium environments for culturing of pre-implantation mouse embryos. Comp Med 2002;52:122-126.PMID: 12022391.

36. Isachenko V, Maettner R, Sterzik K, Strehler E, Kreinberg R, Hancke K, et al. In-vitro culture of human embryos with mechanical micro-vibration increases implantation rates. Reprod Biomed Online 2011;22:536-544.PMID: 21640308.


37. Blake JR, Vann PG, Winet H. A model of ovum transport. J Theor Biol 1983;102:145-166.PMID: 6876839.


38. Heo YS, Cabrera LM, Bormann CL, Shah CT, Takayama S, Smith GD. Dynamic microfunnel culture enhances mouse embryo development and pregnancy rates. Hum Reprod 2010;25:613-622.PMID: 20047936.