Systematic review of the roles of inositol and vitamin D in improving fertility among patients with polycystic ovary syndrome
Article information
Abstract
Polycystic ovary syndrome (PCOS) is a common endocrine and metabolic disorder among reproductive-age women. As a leading cause of anovulatory infertility, it complicates fertility treatments, including in vitro fertilization. The widely accepted 2003 Rotterdam diagnostic criteria for PCOS include sub-phenotypes based on variations in androgen excess, ovulatory dysfunction, and polycystic ovarian morphology. In this systematic review, we examined the impacts of inositol and vitamin D on fertility in PCOS. Adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 guidelines, we used relevant keywords to comprehensively search databases including PubMed, Google Scholar, and MDPI. From an initial pool of 345 articles, 10 met the inclusion criteria. The articles suggest that vitamin D and inositol, particularly myo-inositol and D-chiro-inositol, may represent therapeutic options for PCOS. Vitamin D influences ovarian follicular development, glucose regulation, and insulin sensitivity. When combined with metformin therapy, it is associated with improved menstrual regularity and ovulation. Inositol is crucial for cellular signaling, energy metabolism, glucose regulation, and fertility. This systematic review underscores the importance of investigating inositol and vitamin D within a PCOS management strategy, given the disorder’s prevalence and impacts on fertility and metabolic health. Although these agents show promise, additional research could clarify their mechanisms of action and therapeutic benefits. This review emphasizes the need for exploration of effective treatments to improve the quality of life among individuals with PCOS. Inositol and vitamin D represent potential options, but more studies are required to elucidate their roles in the management of this condition.
Introduction
Polycystic ovary syndrome (PCOS) is potentially the most prevalent endocrine and metabolic disorder among women of reproductive age, affecting between 6% and 20% of this demographic [1]. This condition contributes to about 75% of cases involving anovulatory infertility [2,3]. Individuals with PCOS undergoing in vitro fertilization typically yield a higher number of retrieved oocytes than infertile women without PCOS; however, these oocytes, along with the resulting embryos, are of lower quality and maturity [4]. PCOS is diagnosed based on the presence of androgen excess, ovulatory dysfunction, and/or polycystic ovaries. The 2003 Rotterdam criteria, which were endorsed by a 2012 National Institutes of Health workshop and reaffirmed in 2018, are crucial for this process. These criteria identify sub-phenotypes, which include combinations of androgen excess and ovulatory dysfunction, androgen excess and polycystic ovarian morphology (PCOM), ovulatory dysfunction and PCOM, or all three. Assuming the exclusion of other etiologies, a diagnosis is established when two of the three following conditions are met: hyperandrogenism, oligo-anovulation, or polycystic ovaries as identified by ultrasound [5]. Vitamin D deficiency (VDD), which is often observed in individuals with PCOS, can be addressed with supplementation. This intervention has been shown to reduce levels of anti-Müllerian hormone (AMH) and inflammation. Combined with metformin, vitamin D supplementation can enhance menstrual regularity and ovulation, consequently impacting follicular development and hormone production. Additionally, vitamin D participates in glucose homeostasis through various mechanisms, including the action of receptors in pancreatic beta cells and skeletal muscle, the activity of the 1-α-hydroxylase enzyme that converts 25-hydroxyvitamin D to its active form (1,25-dihydroxyvitamin D), and the presence of a vitamin D response element in the human insulin gene promoter [6]. Inositol, an important substance for female fertility and pregnancy, exists as several isomers, including myo-inositol (MYO) and D-chiro-inositol (DCI). MYO is widely distributed in various tissues, whereas DCI is less common. The ratio of these isomers reflects the energy needs of the body. Inositol is involved in numerous biological processes, including insulin and gonadotropin signaling, energy metabolism, follicle maturation, glucose regulation, cell movement, and neural tube closure. These functions are essential for fertility, pregnancy, and embryogenesis [7]. The objective of this systematic review is to assess the effects of inositol and vitamin D on fertility among individuals with PCOS. Adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines, we conducted comprehensive searches using terms such as “inositol,” “vitamin D,” “infertility,” and “polycystic ovary syndrome” across databases including PubMed, Google Scholar, Cochrane Library, and Multidisciplinary Digital Publishing Institute (MDPI). This review was limited to articles published in English.
Eligibility criteria
1. Inclusion criteria
The selected articles focused on evaluating the impact(s) of inositol and/or vitamin D supplementation on infertility in patients with PCOS: Participants (The studies involved female patients who had been diagnosed with PCOS using established diagnostic guidelines, such as the Rotterdam criteria, and who were of reproductive age); Intervention (The research was required to examine the effects of inositol and/or vitamin D supplementation as a therapeutic approach); Outcomes (Eligible studies were those that reported on fertility or reproductive health outcomes. These could include pregnancy rates, ovulation rates, live birth rates, hormonal profiles—for example, levels of luteinizing hormone [LH] or follicle-stimulating hormone [FSH]—menstrual regularity, or markers of insulin resistance); Language (The studies were required to have been published in English and to be available in full text).
2. Exclusion criteria
The exclusion criteria were as follows: gray literature; studies published in languages other than English; animal studies, in vitro studies, or investigations conducted on non-human subjects; and duplicate publications or redundant data.
Selection process
We employed Endnote (Clarivate Analytics) for article management and the removal of duplicate entries. The screening of titles and abstracts was conducted independently, with any discrepancies addressed through discussion among the co-authors. Subsequently, the full texts of the selected articles were examined, and those meeting the predetermined criteria were chosen for in-depth evaluation. All reviewers reached a consensus regarding article selection.
Quality assessment of the studies
The selected articles were subjected to quality assessment using appropriate tools. The co-authors were actively involved in this evaluation process. For clinical trials, the Cochrane tool was employed. The Newcastle-Ottawa Scale was utilized for observational studies, the A Measurement Tool to Assess Systematic Reviews (AMSTAR) instrument was applied to systematic reviews, and the Scale for the Assessment of Narrative Review Articles (SANRA) checklist was used for narrative reviews [8]. Only those studies that met the established quality standards were incorporated into the review. The same has been described in Table 1.
Results
1. Study identification and selection
A total of 345 relevant articles were identified across six databases. Following the exclusion of 39 duplicate articles, the remaining studies underwent detailed screening of titles and abstracts, leading to the retrieval of the full texts of 22 selected articles. After assessments of eligibility and quality, 10 articles were ultimately chosen for inclusion in the review. The study selection process is depicted as a PRISMA flowchart in Figure 1 [9]. The articles were subjected to eligibility evaluation using appropriate quality appraisal tools. The results of this quality assessment are presented in Figures 2-5 [7,10].
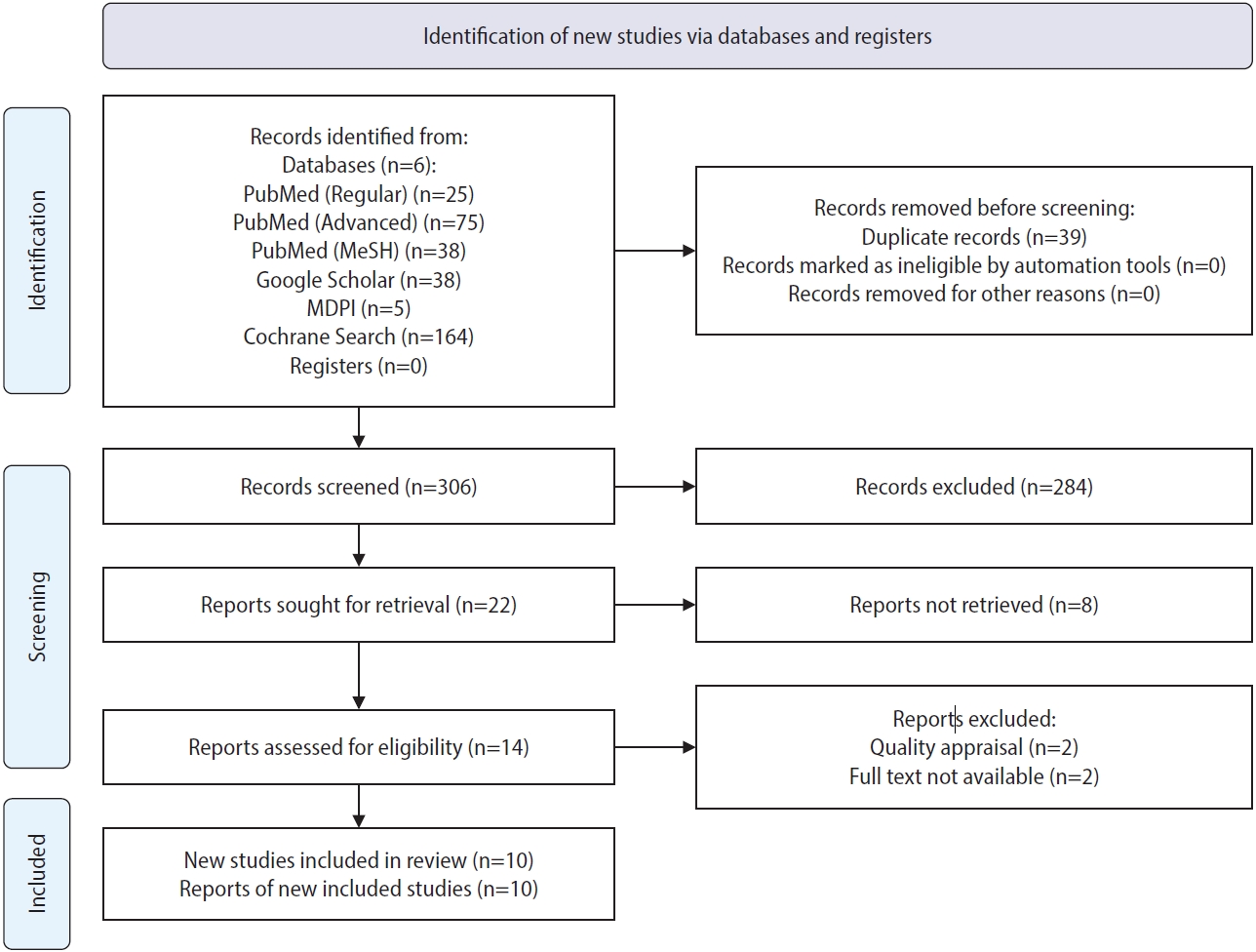
Flowchart of literature review search conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines.
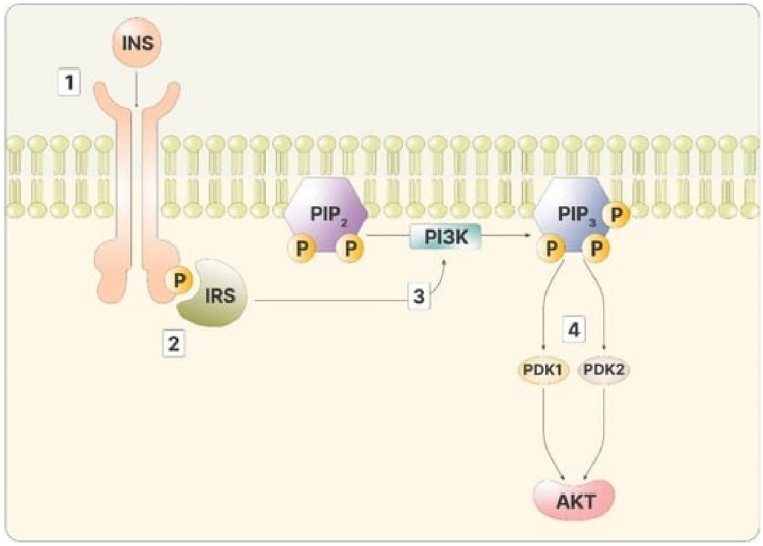
The figure depicts the intracellular cascade stimulated by insulin (INS). (1) INS binding: INS binds to its receptor on the cell surface; (2) insulin receptor substrate (IRS) recognition: IRS recognizes and binds to the phosphorylated insulin receptor; (3) activation of phosphoinositide 3-kinase (PI3K): activated IRS promotes the activity of PI3K, leading to the production of phosphatidylinositol (3,4,5)-trisphosphate (PIPS); (4) activation of PIP3 stimulates both phosphoinositide-dependent kinase 1 (PDK1) and 2 (PKD2), activating protein kinase B (Akt). Adapted from Gambioli et al. [7].
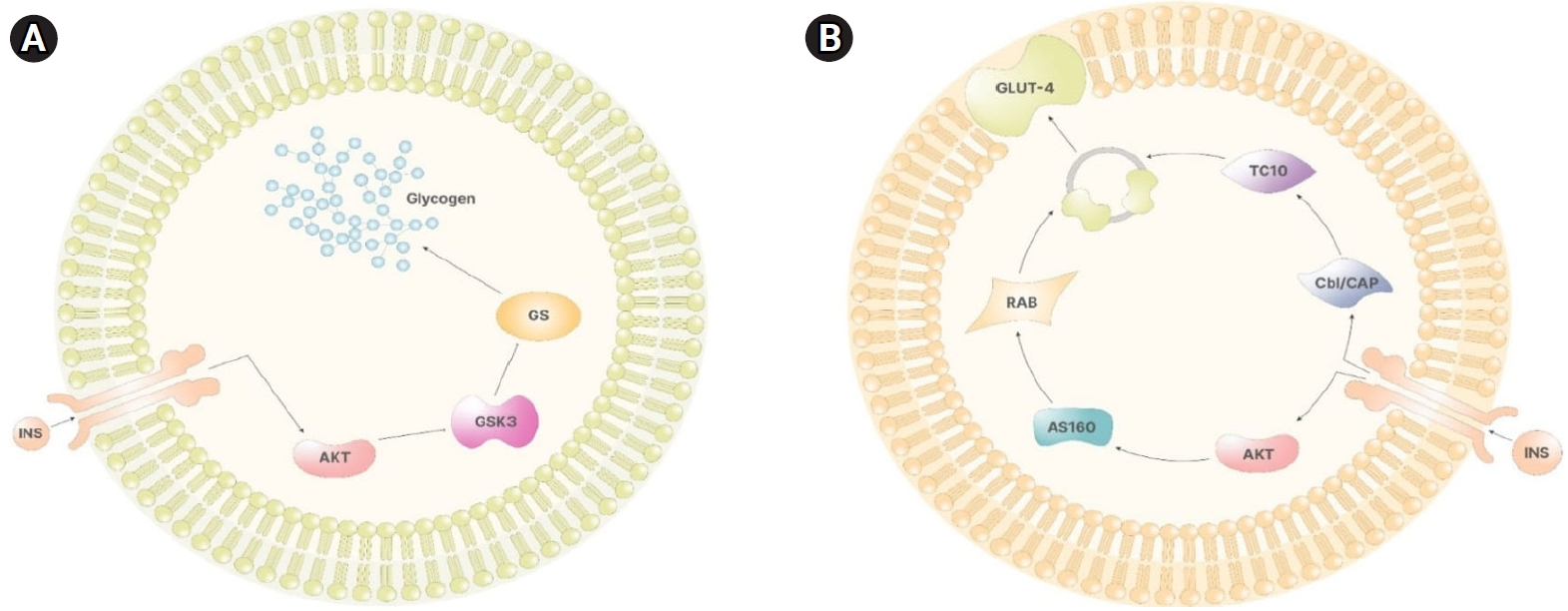
Illustrates the synthesis of glycogen in the liver. (A) Insulin (INS) signaling trigger activating protein kinase B (Akt) activation through phosphatidylinositol (3,4,5)-trisphosphate (PIP3). In the liver cells, Akt inhibits glycogen synthase kinase 3 (GSK3), which, if active, would inhibit glycogen synthase (GS), ultimately promoting glycogen synthesis. (B) In non-storage tissues, Akt activation facilitates AS160 activity. AS160 activates Rab, leading to the formation of vesicles containing glucose transporter 4 (GLUT-4). Simultaneously, the INS receptor activates the Cbl/CAP complex, which, via TC10, facilitates the release of vesicles containing GLUT-4. Adapted from Gambioli et al. [7].

Role of inositol in gonadotropin signaling. (A) Due to its low affinity for adaptor protein, phosphotyrosine interacting with PH domain and leucine zipper 1 (APPL1), the follicle-stimulating hormone (FSH) receptor only initiates APPL1 activation when FSH levels are elevated. This activation, in turn, triggers activating protein kinase B (Akt) and induces phosphoinositide 3-kinase (PI3K) stimulation, resulting in the generation of phosphatidylinositol (3,4,5)-trisphosphate (PIP3). (B) The luteinizing hormone (LH) receptor exhibits limited ability to stimulate the alpha subunit of Gq proteins. Consequently, elevated LH levels prompt the activation of phospholipase C (PLC), which facilitates the cleavage of phosphatidyl-inositol-4,5-bisphosphate (PIP2), generating inositol-1,4,5-trisphosphate (IP3). Adapted from Gambioli et al. [7].
2. Outcomes measured
Of the 10 included studies of individuals with PCOS, three investigated the impact of vitamin D on fertility, while the remainder examined the fertility-enhancing potential of inositol. Consequently, our principal outcome concerned the effects of inositol and vitamin D on fertility in these patients. We also assessed secondary outcomes concerning the improvement of metabolic profiles among women with PCOS. Additionally, several studies addressed the potential of vitamin D and inositol to enhance the outcomes of assisted reproductive technologies (ARTs).
3. Study characteristics
We examined 10 research articles, representing a total of 1,453 participants. These included narrative reviews, randomized controlled trials, observational studies, and one systematic review. Each study incorporated individuals with PCOS as control participants. Seven of these studies explored the effects of inositol on fertility in patients with PCOS. One narrative review discussed the role of vitamin D in reproductive health, its association with metabolic alterations, and its impact on mental health in those with PCOS. Another study addressed the potential for vitamin D to enhance metabolic parameters among these individuals. Tables 2 and 3 summarize the characteristics of the included studies [11-20].
Discussion
1. Endocrine profile of PCOS
PCOS is characterized by elevated androgen production from both the ovaries and the adrenal glands. This hormonal imbalance triggers increased levels of LH and estrogen, predominantly estrone. Individuals with PCOS often exhibit comparatively low levels of sex hormone-binding globulin (SHBG) and high levels of prolactin and insulin, a pattern that is especially common in those who are overweight or obese. Typically, PCOS is associated with elevated LH levels and disrupted FSH release, with an LH-to-FSH ratio greater than 2. This imbalance stimulates theca cells within the ovaries that are sensitive to these hormones, resulting in the overproduction of androgens and disrupting normal follicle development. Furthermore, PCOS is characterized by excess presence of androgens, although the degree of this excess can vary among individuals. While the ovaries are the primary source of increased androgen production, the adrenal glands also contribute. In some cases of PCOS, minor adrenal enzyme deficiencies (such as 21-hydroxylase) or increased adrenal activity due to stress may play a role. The ovaries are the main producers of androstenedione and testosterone, the latter of which is also derived from the conversion of androstenedione, while adrenal secretion is indicated by the level of dehydroepiandrosterone sulfate [21].
Cytochrome P450c17 enzymes play a pivotal role in the production of androgens within the adrenal glands and ovaries. Damage to the adrenal glands or alterations in insulin sensitivity—characterized by heightened insulin resistance and a compensatory rise in hyperinsulinemia—may lead to hyperandrogenism associated with PCOS by augmenting androgen secretion. Furthermore, the enzyme 5α-reductase converts excess testosterone into dihydrotestosterone, fostering an androgenic milieu that, when impacting the skin, can induce hirsutism [17].
Additionally, individuals with PCOS exhibit elevated plasma levels of estrone, a form of estrogen that is approximately 100 times less potent than estradiol. This increase in estrone levels is attributed to the enhanced activity of the enzyme aromatase, which facilitates the conversion of androstenedione into estrogen. Consequently, patients with PCOS tend to have a more estrogenic profile relative to healthy individuals. Such an imbalance may shift the estradiol-to-estrone ratio towards a hyperestrogenic state, potentially increasing the risk of endometrial proliferation and the development of endometrial cancer. The hyperandrogenic state characteristic of PCOS is also known for its suppression of SHBG synthesis, leading to higher levels of free, unbound circulating steroids, predominantly androgens. This elevation in androgens can manifest clinically as hirsutism and acne [7].
2. Role of inositol in improving fertility among patients with PCOS
Inositol comprises a group of natural polyols, which are sugar molecules featuring hydroxyl groups attached to a cyclohexane ring. These substances belong to a category of organic compounds known as cyclohexanols. Such compounds are present in a variety of foods, including fruits, legumes, grains, and nuts. Inositol fulfills multiple roles within cells, contributing to the structure of cell membrane phospholipids, plasma lipoproteins, and the phosphorylated forms found in the nucleus. Consequently, inositol is implicated in numerous cellular functions, such as signal transduction, the regulation of osmolarity, and the modulation of ion channels in adults [22]. Furthermore, MYO is crucial for fetal development and continues to play a major role during the postnatal period, underscoring its importance beginning in the early stages of life [7].
DCI is produced through an epimerization process that alters the position of the C1 hydroxyl group (inositol) on MYO [7]. It functions as a secondary messenger for insulin signaling and is found in relatively high concentrations in adipose tissue, muscle, and liver. This suggests a need for the enzyme nicotinamide adenine dinucleotide (NAD)/nicotinamide adenine dinucleotide hydrogen (NADH) epimerase, which is implicated in tissue differentiation and synthesis. MYO, serving as a secondary messenger, is a critical biomolecule in numerous signaling pathways that are vital for processes such as the formation of cell membranes, lipid synthesis, and hormone regulation. In the ovary and oocyte, these compounds have specific roles. Notably, MYO is crucial for egg development, as it regulates the release of intracellular calcium ions. This isomer comprises approximately 99% of the total inositol pool within the ovary [7].
In a 2022 systematic review and meta-analysis, Sigue and Decena [20] demonstrated improvements in body mass index, menstrual irregularities, homeostatic model assessment of insulin resistance, and AMH levels in two groups: one treated with MYO alone, and the other with a combination of MYO and metformin [14,23]. The administration of MYO as a monotherapy in women with infertility due to PCOS may be an effective substitute for metformin treatment, which is particularly relevant given the association of metformin with gastrointestinal side effects [23].
A randomized trial conducted by Akbari Sene et al. [15] demonstrated the benefits of inositol supplementation in patients with PCOS undergoing ART cycles. The supplementation markedly increased the proportion of metaphase II oocytes relative to the total number of retrieved oocytes. Additionally, it improved fertility rates and resulted in a greater number of high-quality embryos, indicating its potential as an effective intervention for improving ART outcomes in patients with PCOS.
3. Role of inositol in insulin signaling
In the cytoplasm, inositol phosphates are essential for the transmission of insulin signals. This process is initiated when insulin attaches to its receptor, which is a transmembrane tyrosine kinase receptor. The receptor’s intracellular domains phosphorylate one another through kinase activity, thereby increasing their affinity for additional molecules. The phosphorylated insulin receptor then phosphorylates the insulin receptor substrate (IRS) proteins situated within the cell membrane. Phosphorylated IRS serves as a binding site for phosphoinositide 3-kinase (PI3K), which then catalyzes the transformation of phosphatidyl-inositol-4,5-bisphosphate (PIP2) into phosphatidylinositol (3,4,5)-trisphosphate (PIP3). Alternatively, PIP2 can be cleaved by phospholipase C (PLC) to generate inositol-1,4,5-trisphosphate (IP3). An increase in IP3 levels initiates a phosphorylation cascade of cytoplasmic proteins, effectively relaying the signal from the cell membrane to the cell’s interior. Concurrently, PIP3 on the cell membranes activates protein kinase B (Akt) via phosphoinositide-dependent kinase 1 and 2, as illustrated in Figure 2 [7].
In the liver, Akt phosphorylates glycogen synthase kinase 3, inhibiting its repressive impact on glycogen synthase. As a result, following insulin stimulation, the inositol cascade facilitates glycogen synthesis in the liver, as depicted in Figure 3A [7].
Activation of the insulin receptor in various tissues initiates the release of vesicles that contain glucose transporters (specifically, GLUT-4) essential for the cellular uptake of glucose. Akt is crucial in this mechanism, as it directly induces vesicle release by activating proteins such as atypical protein kinase C (PKC-ζ and PKC-λ) and AS160. These proteins, in turn, activate Rab proteins that are involved in vesicle formation. Furthermore, the phosphorylation of the casitas B-lineage lymphoma (Cbl)/Cbl-associated protein (CAP) complex by the insulin receptor indirectly facilitates vesicle release. This is achieved by activating TC10, a member of the Rho family of proteins, which promotes the movement of GLUT-4 vesicles to the plasma membrane (Figure 3B).
4. Role of inositol in gonadotropin signaling
In the natural menstrual cycle, the hypothalamus-pituitary-gonadal axis is of paramount importance, as it stimulates folliculogenesis via FSH and LH. In women, FSH marks the onset of the menstrual cycle, initiating the follicular phase, with its levels staying low until the occurrence of ovulation. At the time of ovulation, FSH reaches its peak, then decreases until menstruation. LH exhibits a comparable trend, remaining at low levels during the follicular phase, experiencing a surge prior to ovulation, and subsequently reverting to baseline levels.
MYO plays a pivotal role in both signaling pathways. The binding of FSH to granulosa cell receptors promotes increased affinity for G proteins and adaptor protein, phosphotyrosine interacting with PH domain and leucine zipper 1 (APPL1). This interaction initiates the activation of PI3K/Akt, which triggers transcriptional regulation and PIP3 production. In thecal cells, LH receptor activation initiates a distinct pathway involving the Gq protein alpha subunit and PLC, culminating in IP3 production. These processes occur both preceding and during ovulation, underscoring the critical role of inositol during this phase [7].
5. Role of vitamin D in PCOS
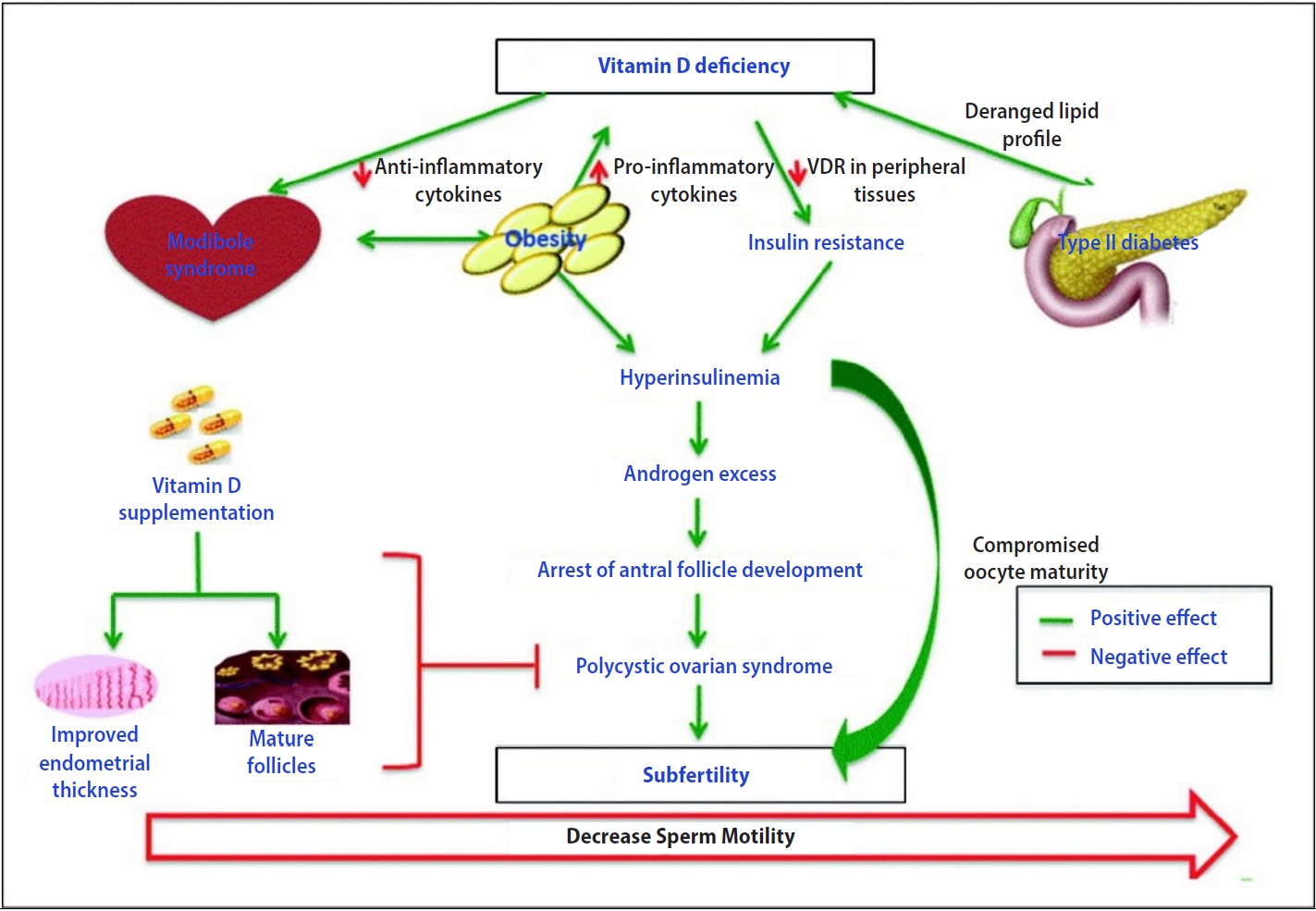
Vitamin D is crucial in regulating female reproductive development by influencing sex hormone steroidogenesis. This role is supported by the presence of the vitamin D receptor and 1-hydroxylase in reproductive tissues, including the ovaries, uterus, placenta, pituitary gland, and hypothalamus. VDD substantially impacts insulin resistance and may play a role in the development of hyperandrogenism in women with PCOS. Specifically, VDD has been linked to decreased levels of SHBG and insulin receptors, which could lead to hyperandrogenism and insulin resistance in those with PCOS.
Moreover, research indicates that women with PCOS exhibit elevated levels of AMH in both follicular fluid and the bloodstream. This change is linked to an increase in the number of follicles and enhanced secretion from granulosa cells. Studies have shown that normalizing vitamin D levels can help regulate AMH secretion and affect follicle selection by reducing AMH messenger RNA expression. In laboratory experiments, treatment of granulosa cells with 1,25-dihydroxyvitamin D3 has been found to increase progesterone production from its precursor, pregnenolone, as depicted in Figure 5. Furthermore, in vivo studies corroborate the notion that vitamin D boosts the secretion of hormones such as estrogen, progesterone, estrone, and insulin-like growth factor-binding protein 1 in ovarian cells.
A 2020 report by Mu et al. [13] demonstrated an association between PCOS and elevated blood sugar levels, as well as hirsutism. The study also indicated that lower vitamin D levels correlate with increased severity of hirsutism in individuals with PCOS. However, the impact of vitamin D on testosterone levels continues to be a subject of debate. While some research has shown no significant alterations in testosterone levels following vitamin D supplementation, other studies have observed a reduction in testosterone levels alongside improved insulin sensitivity. The variability in outcomes may stem from differences in the dosage and timing of vitamin D supplementation.
Endometrial hyperplasia is commonly associated with PCOS, often due to extended menstrual cycles, diminished estrogen activity, and reduced suppression by progesterone. Treatment with vitamin D seems to enhance endometrial thickness and normalize menstrual cycles in patients with PCOS who are deficient in vitamin D. Furthermore, evidence suggests that vitamin D treatment may bolster fertility. Nonetheless, the precise molecular mechanisms underlying these effects warrant further exploration. AMH plays a crucial role in the development of ovarian follicles and is typically present at elevated levels in individuals with PCOS. Research indicates that vitamin D supplementation can decrease AMH concentrations in PCOS patients, which implies a key role for vitamin D in fostering folliculogenesis. Moreover, lower concentrations of vitamin D have been observed in the follicular fluid of PCOS patients. However, additional studies are necessary to elucidate the connection between vitamin D and the process of folliculogenesis in the context of PCOS.
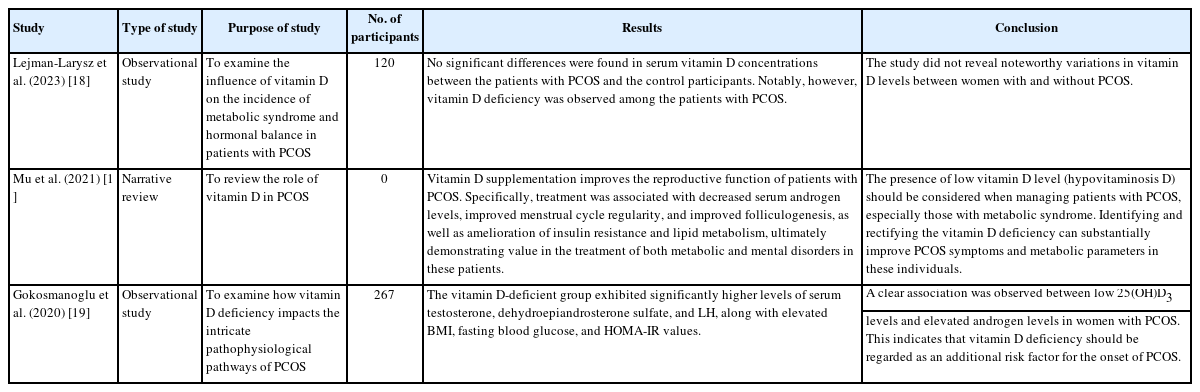
Lejman-Larysz et al. [18] conducted a 2023 study of patients with PCOS and revealed an association between vitamin D levels and two of the five diagnostic criteria for metabolic syndrome. The study identified significant relationships between vitamin D concentrations and both waist circumference and blood pressure. In univariate logistic regression analysis, for each unit increase in vitamin D level among PCOS patients, a 7% decrease was observed in the odds of developing metabolic syndrome.
In 2020, Gokosmanoglu et al. [19] demonstrated an association between VDD and more severe symptoms, as well as alterations in metabolic and hormonal profiles, in patients with PCOS. This research indicates that a deficiency in vitamin D could elevate the risk or contribute to the pathogenesis of PCOS. Furthermore, the link between insulin resistance and PCOS is well-established, and vitamin D has been suggested as a key factor in improving insulin resistance.
6. Limitations
The review was constrained by an insufficient number of studies examining the impact of vitamin D on fertility among patients with PCOS. Additionally, the temporal limitations of the review meant that research conducted prior to 2019 was not included. Moreover, the studies lacked uniformity, as not all of them investigated the same variables and outcomes.
Conclusion
The present systematic review was undertaken to examine the roles of inositol and vitamin D in improving fertility among women with PCOS. The literature suggests that inositol, especially in the forms of MYO and DCI, is integral to cellular functions and may offer advantages in the treatment of PCOS. Research indicates that supplementation with MYO could improve body mass index, mitigate menstrual irregularities, reduce insulin resistance, and normalize AMH levels. A deficiency in vitamin D has been linked to the manifestation of PCOS, and vitamin D supplementation appears beneficial in increasing endometrial thickness and regularizing menstrual cycles in individuals with PCOS and low vitamin D status. Understanding the intricate hormonal and metabolic pathways implicated in PCOS is essential for the diagnosis and effective management of this disorder. Similarly, exploring treatment modalities is critically important for improving the quality of life and health outcomes for those affected. Such treatments hold the promise of alleviating symptoms, enhancing fertility, improving metabolic regulation, and diminishing long-term health complications. Inositol and vitamin D present possible therapeutic options for individuals with PCOS; however, additional studies are required to confirm their mechanisms of action and validate their therapeutic benefits.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: GK (Gitika Katyal). Data curation: GK (Gursharan Kaur). Formal analysis: HA. Funding acquisition: AB. Methodology: GK (Gitika Katyal). Project administration: SK. Visualization: AH. Writing-original draft: DKO. Writing-review & editing: GK, GK, HA, AB, AH, DKO.