|
 |
- Search
| Clin Exp Reprod Med > Volume 51(1); 2024 > Article |
|
Abstract
Objective
The purpose of this study was to use a mouse model to investigate the blastocyst formation rate in vitrified-warmed embryos derived from vitrified-warmed oocytes.
Methods
Metaphase II oocytes obtained from BDF1 mice were vitrified and warmed, followed by fertilization with epididymal sperm. On day 3, a total of 176 embryos, at either the eight-cell or the morula stage, were vitrified-warmed (representing group 1). For group 2, 155 embryos at the same developmental stages were not vitrified, but rather were directly cultured until day 5. Finally, group 3 included day-5 blastocysts derived from fresh oocytes, which served as fresh controls. The primary outcome measured was the rate of blastocyst formation per day-3 embryo at the eight-cell or morula stage.
Results
The rates of blastocyst formation per day-3 embryo were comparable between groups 1 and 2, at 64.5% and 69.7%, respectively (p>0.05). The formation rates of good-quality blastocysts (expanded, hatching, or hatched) were also similar for groups 1 and 2, at 35.5% and 43.2%, respectively (p>0.05). For the fresh oocytes (group 3), the blastocyst formation rate was 75.5%, which was similar to groups 1 and 2. However, the rate of good-quality blastocyst formation in group 3 was 57.3%, significantly exceeding those of group 1 (p=0.001) and group 2 (p=0.023).
Conclusion
Regarding developmental potential to the blastocyst stage, vitrified-warmed day-3 embryos originating from vitrified-warmed oocytes demonstrated comparable results to non-vitrified embryos from similar oocytes. These findings indicate that day-3 embryos derived from vitrified-warmed oocytes can be effectively cryopreserved without incurring cellular damage.
Oocyte cryopreservation is an essential technique for fertility preservation, particularly for female cancer patients who require gonadotoxic treatment and for women who want to delay childbearing [1]. Thanks to massive advancements in cryobiology, oocytes can now be cryopreserved effectively and safely [2-4].
Two methods are used for oocyte cryopreservation: slow freezing and vitrification. Vitrification is generally preferred to slow freezing, as it tends to be less expensive, more convenient, and potentially more effective [5-7]. A 6-year study of 13,847 in vitro fertilization (IVF) cycles using cryopreserved oocytes found that the clinical pregnancy rate per transfer was significantly higher among cycles that employed vitrified oocytes than among those involving slow-frozen oocytes (19.9% vs. 16.0%, p<0.0001) [8].
The existing literature is inconclusive regarding whether vitrified and fresh oocytes are comparable in terms of clinical pregnancy outcomes [9-11]. When an appropriate vitrification protocol was employed, the post-warming oocyte survival rate was observed to reach 90%, with embryo development and clinical pregnancy outcomes approaching those of cycles using fresh oocytes [3]. However, in a separate study, clinical pregnancy rates per transfer in cycles utilizing vitrified oocytes were lower than those in either fresh or frozen embryo transfer cycles using fresh oocytes [8]. Additionally, one investigation revealed that the ongoing pregnancy rate was significantly lower in cycles involving vitrified oocytes compared to those using fresh oocytes, despite similar fertilization, cleavage, and clinical pregnancy rates [12].
When vitrified oocytes are used in an attempt to achieve pregnancy, multiple oocytes are warmed and subsequently fertilized with the partnerŌĆÖs sperm to produce embryos. The physician must determine the number of vitrified oocytes to warm. Current data indicate that the survival rate of oocytes after warming exceeds 90%, and the fertilization rate is reportedly over 75% [13,14]. The proportion of high-quality day-3 embryos derived from zygotes arising from vitrified oocytes has been documented at 33.3% [15]. The rate of blastocyst development from day-3 embryos has been reported to be comparable between vitrified and fresh oocytes (48.7% vs. 47.5%, respectively) [14]. Based on these findings, nine to 10 vitrified oocytes are required to yield two high-quality day-3 embryos, while 15 to 16 vitrified oocytes are needed to produce two blastocysts. However, atypical scenarios are possible, including the re-vitrification of supernumerary embryos or biopsied blastocysts.
When day-3 embryos derived from vitrified-warmed oocytes are themselves vitrified, it remains uncertain whether they sustain more cryo-damage than non-vitrified day-3 embryos derived from similar oocytes. To date, no reports have been published concerning the embryonic development of vitrified day-3 embryos arising from vitrified-warmed oocytes.
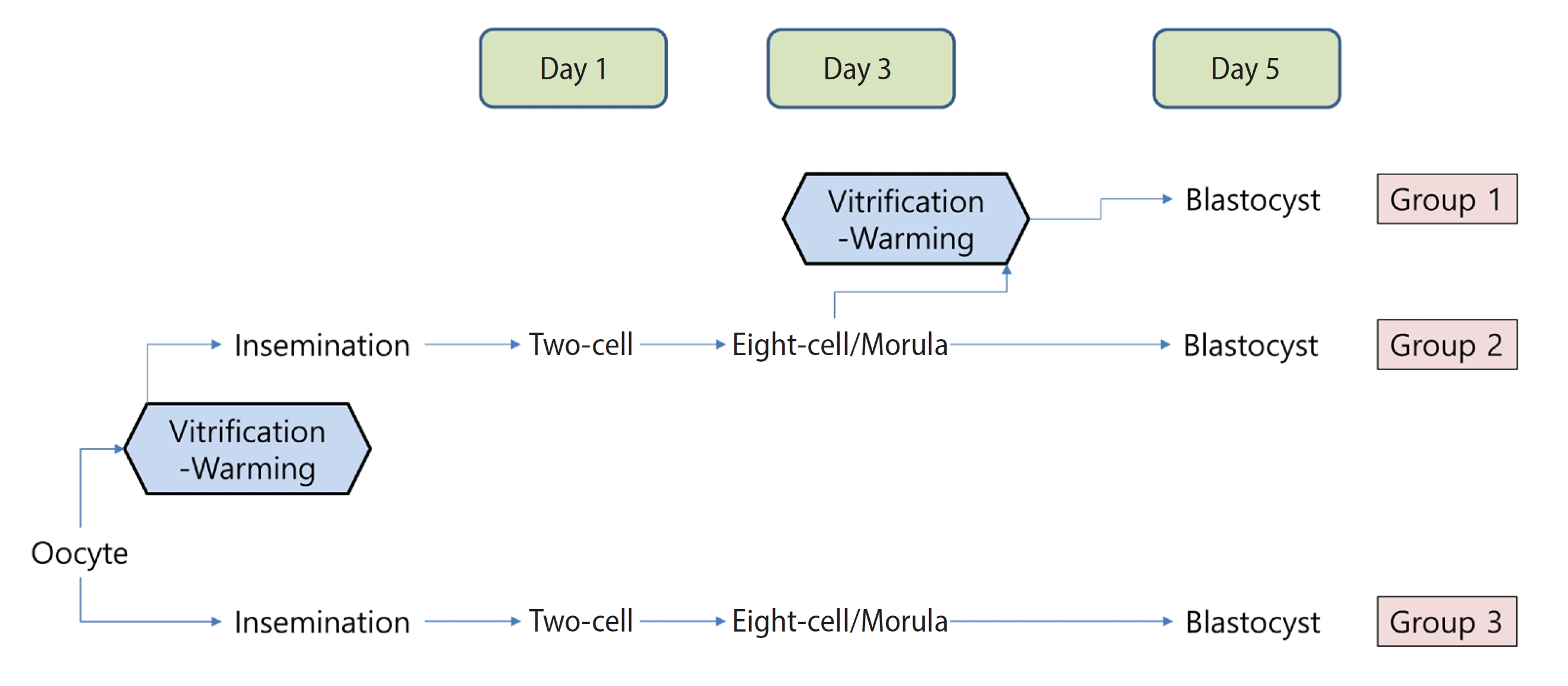
This study was conducted using a mouse model. Day-3 embryos (at the eight-cell or morula stage) were obtained from vitrified-warmed oocytes. These embryos were then either vitrified or left unvitrified, and the day-5 blastocyst formation rate was assessed.
A total of 147 female BDF1 mice, aged 7 to 8 weeks (Orient Co.), were utilized in this study. Additionally, 40 male BDF1 mice of the same age were employed for the collection of epididymal sperm. The mice were housed under a 12-hour light/dark cycle at a temperature of 23 ┬░C and had ad libitum access to food. The Institutional Animal Care and Use Committee (IACUC) of Seoul National University Bundang Hospital granted approval for this research (IACUC number BA-2011-307-097-01). The overall procedures adhered to those of a previous experiment conducted at our center [16].
After a 1-week acclimation period, the female mice were superovulated with an intraperitoneal injection of 7.5 IU of pregnant mareŌĆÖs serum gonadotropin (Daesung Microbiological Labs), followed by a trigger with 5 IU of equine chorionic gonadotropin (eCG; Daesung Microbiological Labs) 48 hours later. Sixteen hours following the eCG injection, the mice were euthanized via cervical dislocation. The bilateral ovaries were excised and immediately placed in 1 mL of L-15 medium (Welgene Inc.) supplemented with 0.4% bovine serum albumin (Sigma-Aldrich). Cumulus oophorus complexes (COCs) were released via mechanical disruption using a syringe needle. The COCs were then transferred to human tubal fluid (HTF; FUJIFILM Irvine Scientific) containing 10% serum substitute supplement (SSS; FUJIFILM Irvine Scientific) and 10 ╬╝L of hyaluronidase (Cook) to enable the collection of metaphase II (MII) oocytes. An MII oocyte, characterized by the extrusion of a polar body, is deemed mature and ready for fertilization or cryopreservation.
The retrieved MII oocytes were immersed in an equilibration solution for 5 minutes. This solution was composed of phosphate-buffered saline (PBS; LB001-02; Welgene Inc.), 20% fetal bovine serum (FBS), 7.5% ethylene glycol (EG; 102466; Sigma-Aldrich), and 7.5% dimethyl sulfoxide (DMSO; D8418; Sigma-Aldrich). The oocytes were then transferred to a vitrification solution, consisting of PBS with 20% FBS, 15% EG, 15% DMSO, and 0.5 M sucrose (S1888; Sigma-Aldrich), for 1 minute. Next, five oocytes were placed onto each polypropylene strip in a Cryotop device (Kitazato Biopharma Co. Ltd). The device was plunged into liquid nitrogen, and the cover was secured using forceps.
The oocytes were warmed within 5 days following vitrification. The warming solutions consisted of PBS supplemented with 20% FBS and three different concentrations of sucrose (1, 0.5, and 0.25 M). The Cryotop device was directly immersed in a 37 ┬░C warming solution including 1 M sucrose for 1 minute, followed by immersion in a warming solution containing 0.5 M sucrose for 3 minutes. Finally, the device was transferred to a warming solution with 0.25 M sucrose for 3 minutes. The recovered oocytes were then moved to a solution of PBS with 20% FBS and subjected to 3 minutes of incubation for washing. After the washing step, the survival rate of the vitrified-warmed oocytes was evaluated.
Surviving oocytes were incubated in 1 mL of HTF with 10% SSS, including 10 ╬╝L of epididymal sperm suspension (0.3 to 2 million/mL), at 37.0 ┬░C in a 5% carbon dioxide (CO2) humidified air environment. Inseminated oocytes were cultured for 24 hours, and two-cell embryos were obtained on the following day (considered day 1).
The two-cell embryos were washed twice using pipetting and then cultured in potassium simplex optimized medium (KSOM; EmbryoMax; EMD Millipore) supplemented with 10% SSS. The culture conditions were maintained at 37 ┬░C with 5% CO2 in a humidified air environment.
On day 3, embryos at the eight-cell or morula stage were selected for vitrification, using the same protocol as that utilized for oocytes. This approach was informed by previous studies indicating minimal differences in blastocyst development competence between vitrified-warmed embryos at these two stages; therefore, we combined them for our analysis [17-19]. The embryos were warmed within 24 hours of vitrification, again using the same method employed for oocytes. After washing, the survival of the vitrified-warmed embryos was assessed; next, they were cultured in KSOM supplemented with 10% SSS at 37 ┬░C and 5% CO2 in a humidified atmosphere for 2 days. On day 5, blastocysts (considered group 1) were obtained. These were categorized into four stages: early, full, expanded/hatching, or hatched.
The early blastocyst stage was characterized by the initial formation of the blastocoel, occupying less than half of the blastocyst volume. A full blastocyst was identified when the blastocoel comprised more than half of the blastocyst volume. An expanded/hatching blastocyst was recognized when the blastocoel was fully developed or herniated through the zona pellucida. Finally, a hatched blastocyst was defined by the presence of trophectoderm outside the zona pellucida.
A portion of the embryos at the eight-cell or morula stage were not vitrified, but rather were directly cultured up to day 5. Blastocysts obtained from this process were used as a control (termed group 2), which consisted of vitrified-warmed oocytes but fresh day-3 embryos.
On day 5, we also obtained blastocysts derived from fresh oocytes, which served as an additional control (group 3; composed of fresh oocytes and fresh day-3 embryos). As with groups 1 and 2, only embryos at the eight-cell or morula stage were selected on day 3 for inclusion in this group.
The schematic flow for the experimental design is presented in Figure 1.
The primary outcome measured was the rate of blastocyst formation per day-3 embryo, specifically at the eight-cell/morula stage. Statistical analyses were conducted using SPSS ver. 25.0 (IBM Corp.), with data expressed as numbers and percentages (%). To assess differences among the three groups, the Kruskal-Wallis test was utilized, followed by a post hoc test if the p-value suggested statistical significance. The chi-square test was employed to compare rates. A p-value of less than 0.05 was considered to indicate significance.
The primary outcomeŌĆöthe rate of blastocyst formation per day-3 embryo at the eight-cell/morula stageŌĆöwas comparable between groups 1 and 2, at 64.5% and 69.7%, respectively (Table 1). Formation rates of good-quality blastocysts (expanded, hatching, or hatched) were also similar for groups 1 and 2, at 35.5% and 43.2%, respectively. For the fresh oocytes (group 3), the blastocyst formation rate was 75.5%, which was similar to those of groups 1 and 2. However, the rate of good-quality blastocyst formation in group 3 was significantly higher, at 57.3%, compared to group 1 (p=0.001) and group 2 (p=0.023).
For the vitrified-warmed oocytes in groups 1 and 2, the oocyte survival rate was 93.4%. The fertilization (two-cell) rate was 42.3%, which was comparable to the rate observed in group 3 (38.9%). On day 3, the rate of eight-cell/morula formation (per two-cell embryo) in groups 1 and 2 combined was significantly lower than in group 3 (24.5% vs. 33.5%, p<0.001). On the same day, a total of 331 eight-cell/morulae were randomly allocated to groups 1 and 2. Within group 1, 176 of these embryos underwent vitrification; after warming, 166 were found to have survived, constituting a survival rate of 94.3%.
The present study showed that the blastocyst formation rate was similar for both vitrified and fresh eight-cell/morula embryos, both of which originated from vitrified-warmed oocytes. Thus, day-3 embryos derived from vitrified-warmed oocytes can be effectively cryopreserved and used without inflicting additional cellular damage. This research is valuable in that it replicated specific re-vitrification scenarios that are likely to arise in human IVF-embryo transfer programs, employing a mouse model to illustrate their effects on blastocyst formation rates.
In this study, oocytes that were vitrified and then warmed formed blastocysts at a rate comparable to that of fresh oocytes. However, the vitrified-warmed oocytes exhibited a significantly lower formation rate of good-quality blastocystsŌĆöthose that were expanded, hatching, or hatchedŌĆöcompared to fresh oocytes. This was the case regardless of the vitrification status of the day-3 embryos.
In a study involving human oocytes [14], the blastocyst formation rate with frozen-thawed oocytes was comparable to that with fresh oocytes (48.7% vs. 47.5%, respectively), which aligns with our findings. However, Cobo et al. [14] did not assess the rate of good-quality blastocyst formation, which could be considered a limitation of their study. In contrast, our mouse study demonstrated a blastocyst formation rate of 69.7% using frozen-thawed oocytes and 75.5% with fresh oocytes. These rates are slightly higher than those reported by Cobo et al. [14]. This discrepancy may be due to our selection criteria, as we specifically chose only good-quality embryos at day 3 (i.e., the eight-cell/morula stage).
In our experiment, the survival rate of vitrified-warmed oocytes was 93.4%, while the survival rate of vitrified-warmed eight-cell/morulae derived from these oocytes was 94.3%. Therefore, we can conclude that the vitrification and warming techniques used in this study were highly efficient.
Freezing oocytes can lead to premature cortical granule release and zona hardening, typically necessitating the use of intracytoplasmic sperm injection to achieve fertilization [20,21]. In our mouse model experiment, however, the fertilization rates of vitrified oocytes were comparable to those of fresh oocytes, despite both being fertilized by conventional insemination. Nonetheless, the rate of eight-cell/morula formation on day 3 was lower for vitrified-warmed oocytes than for fresh oocytes, indicating potential damage incurred during the freezing process.
From these findings, we can postulate that once vitrified-warmed oocytes reach the eight-cell/morula stage, the rate of blastocyst formation is similar, regardless of whether the embryos are frozen at the eight-cell/morula stage. This observation suggests that any damage incurred by the oocyte during freezing was adequately repaired during subsequent fertilization and embryonic development.
In the present study, the overall rate of blastocyst formation was similar between vitrified-warmed oocytes and fresh oocytes. However, the rate of good-quality blastocyst formation was lower in the vitrified-warmed group. This indicates that the damage sustained during the freezing of oocytes may continue to affect development through the blastocyst stage. Essentially, while the developmental capacities of the blastocysts may appear similar, the quality of those derived from vitrified-warmed oocytes might be compromised. In contrast, once vitrified-warmed oocytes reach the eight-cell or morula stage, the rates of both overall and good-quality blastocyst formation were similar regardless of whether the day-3 embryos were frozen. Consequently, the freezing of day-3 embryos originating from vitrified-warmed oocytes does not seem to inflict additional cryo-damage.
The formation of ice crystals, cryoprotectant toxicity, the generation of reactive oxygen species, excessive dehydration, hypothermia, ion imbalance, and altered gene expression are believed to contribute to the damage observed during cryopreservation [22]. Regarding oocyte cryopreservation techniques, relative to slow freezing, vitrification is understood to have a smaller impact on the subsequent embryo. While the carryover effect does not appear to compromise normal cell survival, it may induce subtle functional or molecular alterations that could impair certain developmental processes at later growth stages [22]. Fathi et al. [23] found that although the rate of blastocyst formation did not differ after re-vitrification, both the cell count and the diameter of blastocysts were significantly lower in their re-vitrification group than in the non-frozen or single-frozen groups.
Furthermore, the carryover effect is believed to vary with the developmental stage, including differing results for oocytes and embryos. Our research group previously reported that repeatedly vitrified mouse embryos at the eight-cell stage developed up to the blastocyst stage without cryoinjury [16]. However, another study found that re-vitrification at the eight-cell and blastocyst stages had different impacts on embryonic developmental potential. Specifically, re-vitrification at the blastocyst stage, after initial vitrification at the eight-cell stage, resulted in a lower delivery rate. In contrast, embryos vitrified twice at the eight-cell stage exhibited pregnancy outcomes similar to those of embryos vitrified only once [24]. The mechanisms underlying the differential carryover effects at each stage warrant further investigation.
Although the precise nature of the carryover effect is not fully understood, it is conceivable that critical damage incurred during the vitrification-warming process could account for the observed decrease in cellularity or developmental potential following vitrification and subsequent re-vitrification.
Concerns have been raised regarding the developmental potential and genetic stability of embryos derived from vitrified-warmed oocytes. It is generally understood that the meiotic spindle of oocytes reassembles normally during the warming process. However, this structure is highly sensitive to temperature fluctuations, which may lead to unequal chromosome segregation during meiosis and potentially result in embryo aneuploidy following fertilization [25,26]. The findings of previous studies on this matter are conflicting, necessitating further research.
The strength of our study lies in its consistency in evaluating oocytes and embryonic stages, ensured by the expertise of a skilled embryologist. We made every effort to minimize potential bias by standardizing all conditions, with the exception of the cryostress event pertinent to the experiment. The primary limitation of our study is the inability to observe molecular changes at the gene level that might elucidate the observed phenomena or establish any correlations. For future research, it would be beneficial to investigate the messenger RNA expression levels of genes associated with cryoinjury or antioxidants at the molecular level. Additionally, further studies are warranted to confirm the implantation potential of vitrified-warmed day-3 embryos derived from vitrified-warmed oocytes.
In conclusion, vitrified-warmed day-3 embryos arising from vitrified-warmed oocytes maintain the potential for good embryonic development up to the blastocyst stage when compared with non-vitrified embryos derived from vitrified-warmed oocytes. Hence, day-3 embryos derived from vitrified-warmed oocytes can be efficiently cryopreserved without inducing cellular damage.
Notes
Table┬Ā1.
Oocyte and embryo survival rates and overall development competence for each treatment condition
Values are presented as number (%). A total of 127 mice were utilized for groups 1 and 2, whereas 20 mice were employed for group 3.
a)b)c)The chi-square test was used to compare proportions; d)Significant difference between groups 1 and 3; e)Significant difference between groups 2 and 3; f)Time of randomization for groups 1 and 2.
References
1. Argyle CE, Harper JC, Davies MC. Oocyte cryopreservation: where are we now? Hum Reprod Update 2016;22:440-9.


2. Sciorio R. Cryopreservation of human embryos and oocytes for fertility preservation in cancer and non cancer patients: a mini review. Gynecol Endocrinol 2020;36:381-8.


3. Clark NA, Swain JE. Oocyte cryopreservation: searching for novel improvement strategies. J Assist Reprod Genet 2013;30:865-75.




4. Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril 2013;99:37-43.


5. Smith GD, Serafini PC, Fioravanti J, Yadid I, Coslovsky M, Hassun P, et al. Prospective randomized comparison of human oocyte cryopreservation with slow-rate freezing or vitrification. Fertil Steril 2010;94:2088-95.


6. Levi Setti PE, Porcu E, Patrizio P, Vigiliano V, de Luca R, dŌĆÖAloja P, et al. Human oocyte cryopreservation with slow freezing versus vitrification: results from the National Italian Registry data, 2007-2011. Fertil Steril 2014;102:90-5.


7. Cil AP, Bang H, Oktay K. Age-specific probability of live birth with oocyte cryopreservation: an individual patient data meta-analysis. Fertil Steril 2013;100:492-9.



8. Levi-Setti PE, Patrizio P, Scaravelli G. Evolution of human oocyte cryopreservation: slow freezing versus vitrification. Curr Opin Endocrinol Diabetes Obes 2016;23:445-50.


9. Cobo A, Garrido N, Pellicer A, Remohi J. Six yearsŌĆÖ experience in ovum donation using vitrified oocytes: report of cumulative outcomes, impact of storage time, and development of a predictive model for oocyte survival rate. Fertil Steril 2015;104:1426-34.


10. Garcia-Velasco JA, Domingo J, Cobo A, Martinez M, Carmona L, Pellicer A. Five yearsŌĆÖ experience using oocyte vitrification to preserve fertility for medical and nonmedical indications. Fertil Steril 2013;99:1994-9.


11. Goldman KN, Noyes NL, Knopman JM, McCaffrey C, Grifo JA. Oocyte efficiency: does live birth rate differ when analyzing cryopreserved and fresh oocytes on a per-oocyte basis? Fertil Steril 2013;100:712-7.


12. Potdar N, Gelbaya TA, Nardo LG. Oocyte vitrification in the 21st century and post-warming fertility outcomes: a systematic review and meta-analysis. Reprod Biomed Online 2014;29:159-76.


13. Ata B, Chian RC, Tan SL. Cryopreservation of oocytes and embryos for fertility preservation for female cancer patients. Best Pract Res Clin Obstet Gynaecol 2010;24:101-12.


14. Cobo A, Kuwayama M, Perez S, Ruiz A, Pellicer A, Remohi J. Comparison of concomitant outcome achieved with fresh and cryopreserved donor oocytes vitrified by the Cryotop method. Fertil Steril 2008;89:1657-64.


15. Fu X, Liu X, Li J, Zhang M, Jiang J, Chen Q, et al. An eight year experience of autologous oocyte vitrification for infertile patients owing to unavailability of sperm on oocyte retrieval day. Front Med (Lausanne) 2021;8:663287.



16. Youm J, Kim SK, Jee BC, Kim SH. Embryonic survival, development and cryoinjury of repeatedly vitrified mouse preimplantation embryos. Eur J Obstet Gynecol Reprod Biol 2017;217:66-70.


18. de Sousa Lopes SMC, Mummery CL. Differentiation in early development. In: Lanza R, Gearhart J, Hogan B, Melton D, Pedersen R, Donnall Thomas E, et al. editors. Essentials of stem cell biology. 2nd ed. Elsevier; 2009. p.117-29.
19. Ghandy N, Karimpur Malekshah AA. Which stage of mouse embryos is more appropriate for vitrification? Int J Fertil Steril 2017;10:357-62.

20. Nottola SA, Coticchio G, Sciajno R, Gambardella A, Maione M, Scaravelli G, et al. Ultrastructural markers of quality in human mature oocytes vitrified using cryoleaf and cryoloop. Reprod Biomed Online 2009;19 Suppl 3:17-27.


21. Ghetler Y, Skutelsky E, Ben Nun I, Ben Dor L, Amihai D, Shalgi R. Human oocyte cryopreservation and the fate of cortical granules. Fertil Steril 2006;86:210-6.


22. Jia QP, Sun WQ. Perspective: cryopreservation of human oocytes and the ŌĆścarryoverŌĆÖ effect on early embryo development. Cryo Letters 2021;42:120-8.

23. Fathi R, Valojerdi MR, Yazdi PE, Ebrahimi B, Alipour H, Hassani F. Development of 4-cell mouse embryos after re-vitrification. Cryobiology 2012;64:23-6.


24. Li J, Xiong S, Zhao Y, Li C, Han W, Huang G. Effect of the re-vitrification of embryos at different stages on embryonic developmental potential. Front Endocrinol (Lausanne) 2021;12:653310.



- TOOLS