 |
 |
- Search
| Clin Exp Reprod Med > Epub ahead of print |
Abstract
Objective
Bis-[4-chlorophenyl]-1,1,1-trichloroethane (DDT), one of the most widely used synthetic pesticides, is an endocrine-disrupting chemical with the potential to interfere with the human reproductive system. The effects of DDT and one of its metabolites, p,p╩╣-DDT, on human endometrial stromal cells (ESCs) and health outcomes remain unknown. In this study, we investigated whether p,p╩╣-DDT induces an imbalance in cell proliferation and apoptosis in human ESCs via oxidative stress.
Methods
We assessed apoptosis in ESCs by quantifying the expression of markers associated with both intrinsic and extrinsic pathways. Additionally, we measured levels of reactive oxygen species (ROS), antioxidant enzyme activity, and estrogen receptors (ERs). We also examined changes in signaling involving nuclear factor kappa-light-chain-enhancer of activated B cells.
Results
Following treatment with 1,000 pg/mL of p,p╩╣-DDT, we observed an increase in Bax expression, a decrease in Bcl-2 expression, and increases in the expression of caspases 3, 6, and 8. We also noted a rise in the generation of ROS and a reduction in glutathione peroxidase expression after treatment with p,p╩╣-DDT. Additionally, p,p╩╣-DDT treatment led to changes in ER expression and increases in the protein levels of phosphatidylinositol 3-kinase (PI3K), phospho-protein kinase B (phospho-AKT), and phospho-extracellular signal-regulated kinase (phospho-ERK).
The human endometrium plays a crucial role in the proper functioning of the female reproductive system. Its structure is delicately regulated by ovarian sex hormones and exhibits considerable architectural modifications during each menstrual cycle [1,2]. The shedding of the endometrium during menstruation is the result of arteriole contractions that are regulated by estrogen and progesterone, leading to ischemic necrosis in the functional layers of the endometrium. Studies have revealed the presence of apoptotic bodies and DNA laddering during the late secretory and menstrual phases of the cycle, suggesting that apoptosis may be a key factor not only in menstruation, but also in various pathological conditions [3-6].
Bis-[4-chlorophenyl]-1,1,1-trichloroethane (DDT) was one of the most extensively used synthetic pesticides until the 1970s, and it was widely utilized to protect military personnel from malaria during World War II [7]. DDT is also recognized as an endocrine-disrupting chemical (EDC) that mimics natural hormone activity and can interfere with the human endocrine system; this disruption leads to adverse effects on reproductive health, pregnancy outcomes, puberty development, and immune function, while increasing the risk of hormone-dependent cancers [8,9]. In the United States, the use of DDT was banned in 1972 due to its detrimental impact on both the environment and human health. The presence of DDT and its metabolites in human tissues, coupled with the discovery of their prolonged half-lives, has prompted ongoing research into their health effects in humans [10,11]. Recent studies have indicated that DDT can accumulate in body fat over extended periods and become biomagnified within the food chain [12-14].
The technical mixture of DDT consists primarily of two isomers: p,p╩╣-DDT (approximately 85%) and o,p╩╣-DDT (15%) [15]. The o,p╩╣-isomer is recognized as the most estrogenic component of DDT. The binding capacity of o,p╩╣-DDT to estrogen receptors (ERs) in reproductive tissues is 100 times greater than that of p,p╩╣-DDT, leading to the sequential activation of these receptors [15]. Studies have indicated that DDT is associated with reduced mean luteal phase length, spontaneous abortion, early age at menopause, and reduced duration of lactation [16-20]. Furthermore, a positive, monotonic exposure-response association has been observed between preconception levels of total serum DDT and the risk of early pregnancy loss [21].
Apoptosis, or programmed cell death, is a key process in the development and homeostasis of multicellular organisms, functioning as a natural cell death mechanism. The induction of apoptotic cell death by a variety of external and internal signals has been documented in the contexts of certain diseases [22,23]. Oxidative stress, characterized by an imbalance between the production of reactive oxygen species (ROS) and the bodyŌĆÖs antioxidant defenses, plays a pivotal role in apoptosis. This stress leads to a series of events, including damage to cellular lipids, proteins, and DNA [24]. Increased oxidative stress has been noted in various reproductive disorders involving the endometrium, such as endometriosis, endometrial hyperplasia, and miscarriage [12,13]. Certain EDCs are known to promote oxidative stress in human endometrial stromal cells (ESCs), resulting in abnormal cellular responses [12,13].
To date, few studies have been published that demonstrate the impact of DDT on the human endometrium through oxidative stress, along with associated health outcomes [25-28]. Given the long half-life of DDT, we hypothesized that p,p╩╣-DDT could exert a prolonged toxic influence on the human endometrium. Our research was conducted to determine whether p,p╩╣-DDT induces oxidative stress and apoptosis in human ESCs, as well as to explore the influence of p,p╩╣-DDT on antioxidative enzymes and caspase pathways to elucidate the pathogenesis of related diseases. Furthermore, recognizing that p,p╩╣-DDT is an EDC with estrogenic properties [29], we evaluated the potential mechanisms involving ERs and the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-╬║B) pathway in the human endometrium.
Endometrial samples (n=9) were obtained from reproductive-age women (mean age, 35.7┬▒1.9 years) with a normal menstrual cycle who underwent hysterectomy due to carcinoma in situ of the cervix. These women had no signs of endometrial abnormalities, intramural myomas, or adenomyosis and had not taken any hormonal medications in the preceding 3 months. All samples were histologically confirmed to be free of disease and in the proliferative phase. Each patient provided written informed consent using standardized forms, and the study protocols received approval from the Review Board for Human Research of Dong-A Medical Center (DAUHIRB-17-195). ESCs were extracted from the fresh endometrial tissues. The tissues were finely minced, and the cells were dispersed via incubation in HankŌĆÖs balanced salt solution with added 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES; 25 mM), antibiotics (1 dose), collagenase (2 mg/mL), and DNase (0.2 mg/mL). This was followed by 20 minutes of incubation at 37 ┬░C with agitation. The dispersed endometrial cells were then separated by filtration through a 70-╬╝m sieve. The endometrial glandular epithelium was retained within the sieve, while the dispersed ESCs passed into the filtrate. Purified ESCs were pelleted using centrifugation at 500 ├Śg for 5 minutes, then suspended in HamŌĆÖs F-12/DulbeccoŌĆÖs minimal essential medium supplemented with antibiotics-antimycotics (1%, v/v) and fetal bovine serum (10%, v/v). The initial medium was replaced once the cells had fully adhered; thereafter, the medium was refreshed every 2 to 3 days. Subculturing occurred when the cells reached 80% to 100% confluence on the culture plate. The medium was aspirated, the culture was washed with phosphate-buffered saline (PBS), and the cells were trypsinized with 0.5% trypsin/0.2% ethylenediaminetetraacetic acid (EDTA). Passages four to 10 of these cultures were utilized for all experiments.
The 2,5-diphenyl-2H-tetrazolium bromide (MTT) assay was employed to assess cell viability based on the formation of a blue formazan product by dehydrogenase enzymes present in active cells. Cells were seeded at a density of 5├Ś103 cells/mL in a 96-well plate and treated with concentrations of p,p╩╣-DDT ranging from 1 to 1,000,000 pg/mL. They were then cultured at 37 ┬░C in a humidified environment containing 5% CO2 for a period of 1 to 3 days. Subsequently, MTT solution (5 mg/mL in PBS) was loaded into each well, and the plate was incubated at 37 ┬░C for 4 hours. The absorbance was measured using an enzyme-linked immunosorbent assay reader (BioTek Instruments Inc.) at a wavelength of 495 nm. The data were expressed as optical density units.
The ROS-sensitive probe 2,7-dichlorofluorescein diacetate (DCF-DA) was utilized to measure intracellular ROS production. For the purpose of fluorescence-activated cell sorting, cells were harvested and stained in PBS with 10 ╬╝M DCF-DA for 30 minutes at 37 ┬░C in the dark. Next, the cells were treated with p,p╩╣-DDT or a combination of p,p╩╣-DDT with 0.01 ╬╝M fulvestrant (ICI 182,780) for an additional 30 minutes at 37 ┬░C. Total free radicals were measured via spectrofluorimetry using a flow cytometer (Beckman Coulter Inc.). The levels of free radicals were determined by calculating the mean fluorescence intensity (x-mean value) for each sample. For fluorescence microscopy, cells were stained with DCF-DA for 30 minutes at 37 ┬░C, followed by three PBS washes, and then subjected to p,p╩╣-DDT treatment with or without ICI for 30 minutes as previously described. To estimate the number of attached cells, the cells were washed with PBS and then stained with 4 ╬╝g/mL Hoechst 33342 for 5 minutes. Observations were made using a flexible confocal microscope (Zeiss).
Gene expression patterns were analyzed using real-time quantitative polymerase chain reaction (qPCR). Cells treated with p,p╩╣-DDT were harvested, and total RNA was extracted using FavorPrep Tri-RNA Reagent (FAVORGEN Biotech Co.). Complementary DNA was synthesized from 0.2 ╬╝g of total RNA using Maxime RT PreMix (iNtRON bio). Real-time PCR was conducted in accordance with the instructions of the SYBR Green qPCR Master Mix with Low Rox (SMARTGENE; Samjung Bioscience) and was performed on an Applied Biosystems 7000 real-time PCR system (Life Technologies).
Real-time qPCR was also employed to analyze the messenger RNA (mRNA) expression levels of hormone receptors, antioxidative enzymes, and apoptotic factors. The specific primers utilized for the amplification of each gene are detailed in Supplementary Table 1. Gene expression was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. Each experiment was conducted in triplicate and repeated three times.
For the analysis of total protein, cells were harvested and lysed using a lysis buffer that included protease inhibitor (Sigma-Aldrich) and phosphatase inhibitor cocktail III (Calbiochem). For nuclear protein analysis, cells were lysed in 100 ╬╝L of hypotonic buffer, which contained 10 mM HEPES/potassium hydroxide (KOH), 2 mM MgCl2, 0.1 mM EDTA, 10 mM KCl, and a single dose of protease inhibitor, at a pH of 7.9. This mixture was then kept on ice for 10 minutes, vortexed, and centrifuged at 15,000 ├Śg for 30 seconds. The resulting pellets were suspended in 50 ╬╝L of ice-cold saline buffer, comprising 50 mM HEPES/KOH, 50 mM KCl, 300 mM NaCl, 0.1 mM EDTA, 10% glycerol, and a single dose of protease inhibitor, at a pH of 7.9. This suspension was maintained on ice for 2 hours, vortexed, sonicated for 30 seconds, and then centrifuged at 15,000 ├Śg for 5 minutes at a temperature of 4 ┬░C.
The protein lysates underwent separation via sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were subsequently transferred onto a nitrocellulose membrane. We procured primary antibodies specific to protein kinase B (AKT), extracellular signal-regulated kinase (ERK), caspase-3, and caspase-9 from Cell Signaling Technology. Antibodies for ER-╬▒, ER-╬▓, NF-╬║B, I╬║B, B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax), and ╬▓-actin were obtained from Santa Cruz Biotechnology. For immunodetection, we used an enhanced chemiluminescence peroxidase substrate solution supplied by ELPI Biotechnology. The data were expressed as the ratio of the protein of interest to ╬▓-actin intensity. Each experiment was conducted in triplicate and repeated three times. The antibodies were diluted at a ratio of 1:1,000 for primary antibodies and 1:2,000 for secondary antibodies.
To analyze apoptosis, cells underwent staining with annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI), utilizing the FITC Annexin V Apoptosis Detection Kit I (BD Biosciences). Each experiment was conducted in triplicate and repeated three times. Cells were cultured in 6-well plates and subjected to treatment with p,p╩╣-DDT, with or without ICI, for a duration of 72 hours. Spectrofluorimetric analysis of the staining profiles was performed using a flow cytometer (Beckman Coulter Inc.). For the assessment of DNA fragmentation, cells stained with 4ŌĆ▓,6-diamidino-2-phenylindole (DAPI) were examined under a fluorescence microscope (Zeiss).
Cells cultured on cover slides were fixed with 2% paraformaldehyde for 20 minutes, followed by three washes with PBS with Tween (PBST). The cells were then blocked using PBS containing 10% serum and Triton X-100 for 1 hour at room temperature. Subsequently, the cells were incubated with rabbit anti-ER alpha antibody and NF-╬║B overnight at 4 ┬░C. After washing three times with PBST, the cells were incubated for 45 minutes at room temperature with secondary antibodies that were diluted in the blocking buffer. These secondary antibodies included FITC-mouse anti-rabbit immunoglobulin G (IgG) and mouse anti-rabbit IgG-TR (Santa Cruz Biotechnology). The cells were incubated in DAPI (1 ╬╝g/mL) staining solution for 5 minutes in the dark and washed in PBST before being mounted in an anti-fade mounting solution (Invitrogen). Imaging was performed using a flexible confocal microscope (Zeiss).
The data were analyzed using MedCalc statistical software (MedCalc Software Ltd.), with all results presented as means accompanied by their standard errors. For the MTT assay, a Student t-test was conducted. For the additional analyses, one-way analysis of variance was employed, followed by the Dunnett multiple comparisons test. A probability p-value of less than 0.05 was considered to indicate statistical significance.
The viability of ESCs was evaluated following in vitro treatment with p,p╩╣-DDT. We employed the MTT assay to assess cell viability across a range of p,p╩╣-DDT concentrations (0, 10, 100, and 1,000 pg/mL) at three different time points: 24, 48, and 72 hours. The ESCs remained viable at all concentrations examined in this study (results not shown), with no observed toxicity in the cultured ESCs at any concentration. Consequently, subsequent experiments were conducted using the cells that had been treated for 72 hours at each specified concentration.
To investigate apoptosis, ESCs were treated with p,p╩╣-DDT at concentrations of 0, 10, 100, 1,000 pg/mL, as well as with 1,000 pg/mL of p,p╩╣-DDT with ICI. The proportions of live, apoptotic, and necrotic cells were reported as percentages relative to the control for each treatment group. The percentages of live cells at concentrations of 0, 1,000, and 1,000 pg/mL with ICI were 88.2%, 60%, and 75.9%, respectively. The proportions of apoptotic cells (quadrants D2+D4) at these concentrations were 11.2%, 38.6%, and 23.8%, respectively. The proportions of necrotic cells at the same concentrations were 0.6%, 1.4%, and 0.3%, respectively. These data indicate an increased percentage of apoptotic and necrotic cells at a concentration of 1,000 pg/mL, which was mitigated by the incorporation of ICI treatment (Figure 1A).
The histogram (Figure 1Ba) displays the percentages of necrotic, early apoptotic, and late apoptotic cells at each concentration. The total percentage of apoptotic cells was significantly elevated at concentrations of 100 and 1,000 pg/mL, an effect that was reversed by ICI treatment (Figure 1Bb).
Using reverse transcription-PCR, the mRNA expression levels of key components in the intrinsic apoptosis pathway, such as B-cell lymphoma-extra large (Bcl-XL), Bcl-2, and Bax, were quantified in human ESCs exposed to varying concentrations of p,p╩╣-DDT. The antiapoptotic Bcl-2 exhibited a decrease, while the proapoptotic regulator Bax was significantly elevated at a concentration of 1,000 pg/mL (Figure 2A). Additionally, all members of the caspase family examined (specifically, caspases 3, 6, and 8), which are involved in the extrinsic pathway and can trigger the caspase cascade, displayed increased levels at 1,000 pg/mL (Figure 2B). Classic signs of apoptosis, including cell shrinkage, blebbing of the plasma membrane, cell detachment, externalization of phosphatidylserine, nuclear condensation, and ultimately DNA fragmentation, were observed in the p,p╩╣-DDTŌĆōtreated human ESCs. These findings were confirmed using flow cytometry and fluorescence microscopy (Figure 2C).
Protein levels of Bax and Bcl-2, indicative of the intrinsic apoptosis pathway, were elevated at a concentration of 1,000 pg/mL. Both of these increases were reversed by ICI treatment (Figure 2Da). Similarly, the protein levels of FAS, a marker of the extrinsic apoptosis pathway, along with caspase-9ŌĆöan initiator caspase that activates downstream caspases through cleavageŌĆöwere also high at 1,000 pg/mL. These elevations were likewise mitigated by ICI treatment (Figure 2Db).
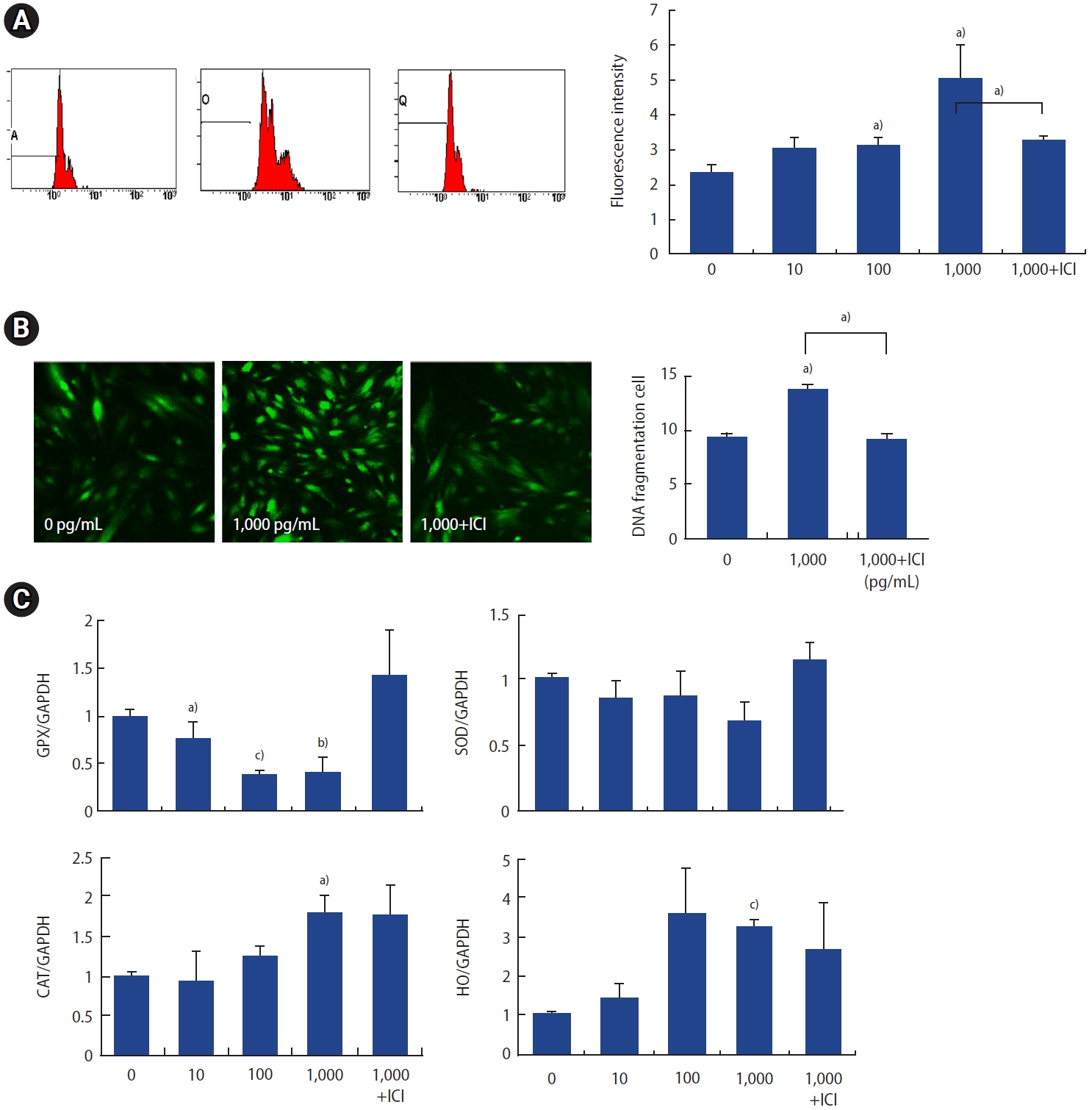
To assess free radical production, cells were loaded with 10 ┬ĄM DCF-DA and subsequently treated with p,p╩╣-DDT. Cells not treated with p,p╩╣-DDT served as negative controls. Exposure of ESCs to p,p╩╣-DDT at concentrations of 100 and 1,000 pg/mL for 30 minutes led to a significant elevation in DCF fluorescence (measured in relative fluorescence units) relative to the control group (Figure 3A). Confocal microscopy revealed pronounced changes in ROS production at the 1,000 pg/mL concentration and following ICI treatment (Figure 3B). Analysis of antioxidative enzyme levels revealed that cells exposed to p,p╩╣-DDT exhibited a significant reduction in glutathione peroxidase (GPX) expression at both 100 and 1,000 pg/mL concentrations compared to the negative control (Figure 3C).
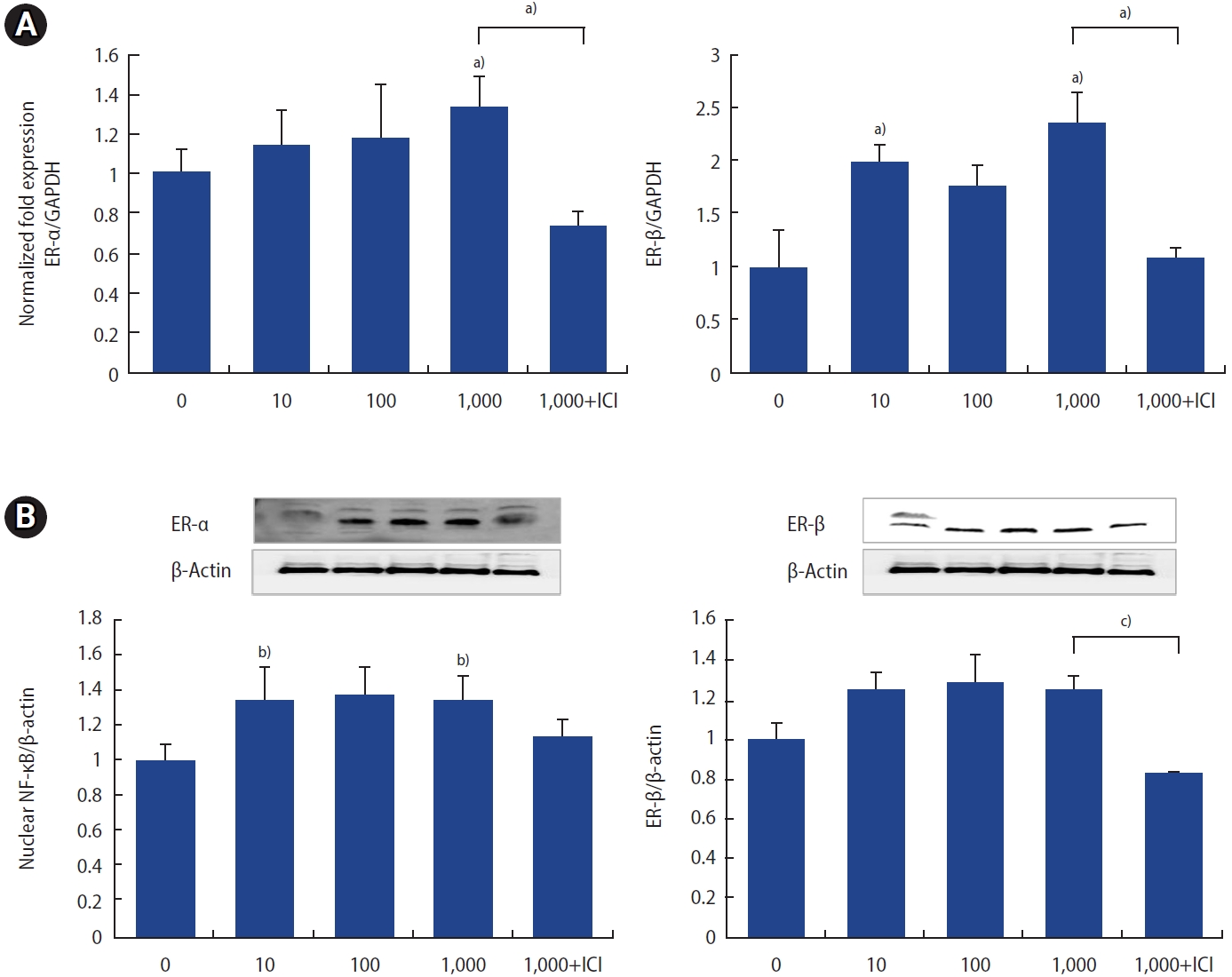
For the assessment of mRNA expression, all values were normalized to GAPDH as a loading control. The expression levels of both ER-╬▒ and ER-╬▓ mRNA were elevated at a concentration of 1,000 pg/mL of p,p╩╣-DDT, and both increases were reversed by ICI treatment (Figure 4A).
The protein levels of ER-╬▒ and ER-╬▓ were elevated across all concentrations, with significant increases observed at 1,000 pg/mL (Figure 4B). Collectively, these findings suggest that p,p╩╣-DDT may upregulate the levels of both ER-╬▒ and ER-╬▓ in human ESCs.
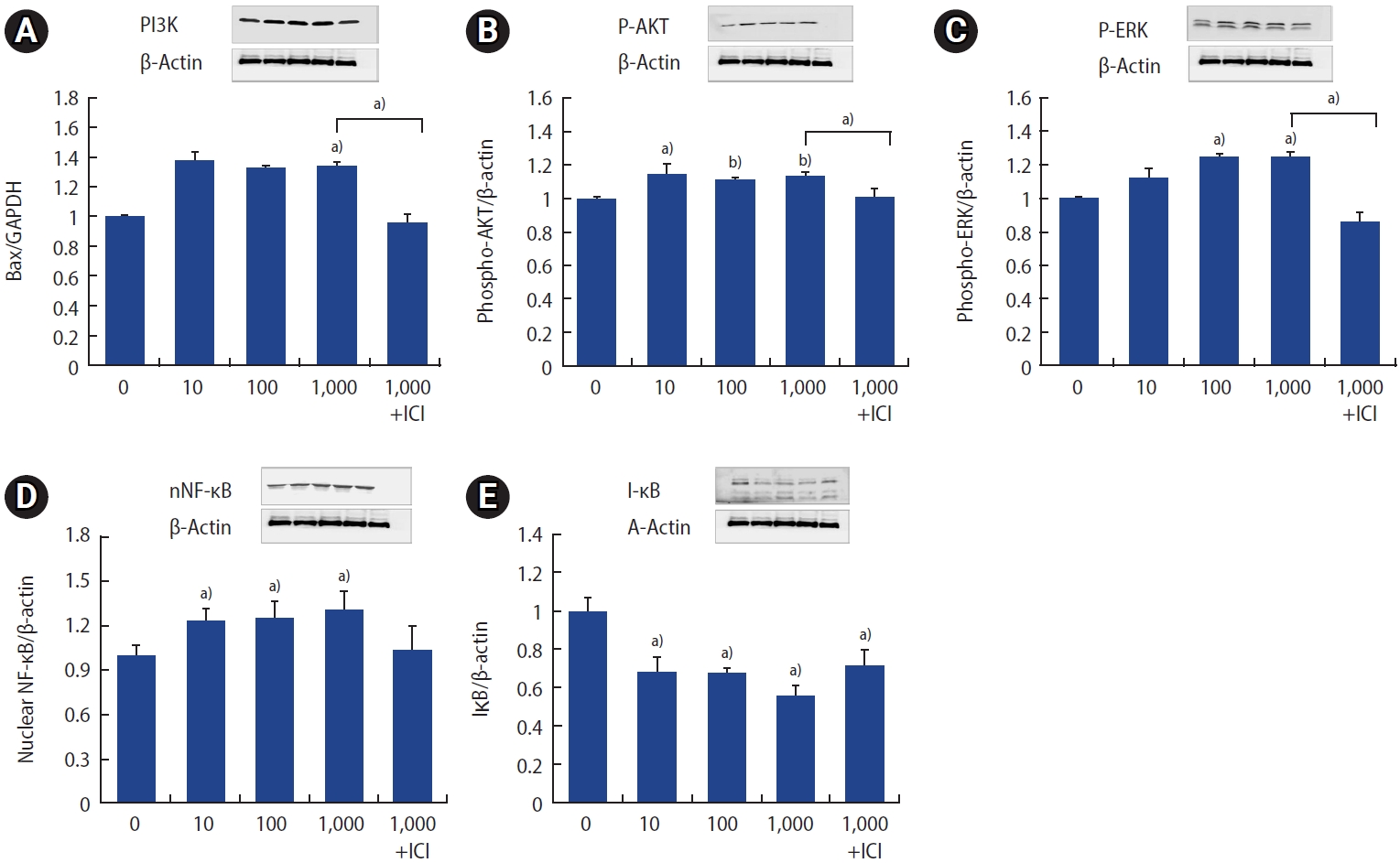
Cells exposed to p,p╩╣-DDT exhibited elevated protein levels of phosphatidylinositol 3-kinase (PI3K) phospho-AKT, and phospho-ERK (Figure 5A-5C). To elucidate the mechanism underlying the effects of p,p╩╣-DDT, we assessed the levels of NF-╬║B/I╬║B using western blot analysis. Treatment with p,p╩╣-DDT at concentrations of 10, 100, and 1,000 pg/mL resulted in elevated NF-╬║B levels and induced the degradation of I╬║B. This effect was reversed by treatment with ICI (Figure 5D, 5E). Therefore, p,p╩╣-DDT appears to activate the PI3K/AKT and ERK signaling pathways, which play a role in the regulation of apoptotic signaling in ESCs.ŌĆā
In this study, we found that p,p╩╣-DDT, a chemical known to disrupt endocrine function, plays a role in the apoptotic pathway of human ESCs via oxidative stress. This involvement is evidenced by upregulation of proapoptotic genes, downregulation of antiapoptotic genes, enhanced generation of ROS, suppressed expression of antioxidant enzymes, and changes in ER expression.
Numerous studies have explored the potential link between exposure to p,p╩╣-DDT and reproductive diseases [17,20,21]. However, the relationships between p,p╩╣-DDT exposure and apoptosis, oxidative stress, and hormone receptor activity in human ESCs have yet to be thoroughly examined. ROS are recognized as normal cellular metabolic byproducts, yet an overabundance of ROS has been observed in a variety of human disorders, including cancer, cardiovascular, neurodegenerative, and endocrine diseases [30]. Furthermore, ROS are implicated in promoting tumor development and progression, as well as contributing to resistance to treatment by influencing apoptotic pathways, which can lead to DNA damage [30]. Our research aimed to investigate whether exposure to p,p╩╣-DDT induces apoptosis in ESCs, and to determine if this is associated with an increase in ROS production and a decrease in antioxidant enzyme levels.
We assessed the mRNA expression levels of both intrinsic and extrinsic apoptotic markers. The expression of Bcl-2, an antiapoptotic marker, markedly decreased, while proapoptotic markers, such as Bax, exhibited a substantial increase upon exposure to p,p╩╣-DDT. This exposure subsequently triggered the release of cytochrome C. Caspases play a pivotal role as mediators of apoptosis and inflammation. Specifically, caspase-6 acts as the direct activator of caspase-8 within the cytochrome-CŌĆōinduced apoptosis pathway. Caspase-3 is known to induce hallmark apoptotic characteristics, including DNA fragmentation and cell death across various tissues [23,24]. Cytochrome C and deoxyadenosine triphosphate associate with apoptotic protease activating factor 1 (APAF-1) to form a multimeric complex. This complex then recruits and activates procaspase-9, an executioner protease that mediates apoptosis, which subsequently activates caspase-3, culminating in cell apoptosis (Figure 6). Our findings indicate significant elevations in the mRNA expression of caspase-3, -6, and -8 at a concentration of 1,000 pg/mL of p,p╩╣-DDT, supporting the induction of apoptosis in human ESCs through p,p╩╣-DDT exposure. These results align with those of a prior study, which reported a lower Bcl-2 level along with a higher Bax level and apoptotic index in trophoblastic cells from patients with early unexplained miscarriage. This suggests a potential link between excessive apoptosis in trophoblastic cells and pregnancy loss [31]. A morphological examination further revealed chromatin condensation and DNA fragmentation in ESCs treated with p,p╩╣-DDT. Additionally, flow cytometry analysis using annexin V-FITC/PI staining demonstrated an elevated percentage of apoptotic cells at higher p,p╩╣-DDT concentrations.
In addition to apoptotic markers, we investigated the impact of oxidative stress by quantifying the generation of ROS and the activity of antioxidant enzymes. We identified an increase in ROS production and a decrease in antioxidant enzyme levels, suggesting that ROS induced by p,p╩╣-DDT may exceed the capacity of the intracellular antioxidant system. This observation aligns with prior research conducted on various cell types [32,33].
p,p╩╣-DDT is a recognized EDC that can dynamically alter various cellular pathways and disrupt normal cellular functions. In humans, estrogen exerts its biological effects by binding to ERs in target tissues. Given that p,p╩╣-DDT exhibits estrogenic effects, it can bind to ERs and mimic the action of estrogens. ERs are typically involved in the regulation of reproductive processes, including fertility, ovulation, prolactin release, oocyte development, and mammary gland function [29,34]. Specifically, ER-╬▒ is crucial for the menstrual cycle, influencing the proliferation, secretion, apoptosis, and shedding of the endometrial lining [35]. Additionally, ER-╬▒ activation can stimulate intracellular signaling pathways such as the mitogen-activated protein kinase (MAPK) and PI3K pathways, which have been implicated in the regulation of apoptosis, cell survival, and the cell cycle [36]. Notably, the activation of the PI3K/AKT signaling pathway is linked to relatively aggressive forms of endometrial cancer and poor prognosis [36,37]. Our research demonstrated that p,p╩╣-DDT exposure leads to an increase in both ER-╬▒ and ER-╬▓ expression in human ESCs. Following p,p╩╣-DDT treatment, we observed a rise in ER mRNA expression and protein levels in ESCs, which was associated with changes in oxidative stress, apoptosis pathways, and PI3K/AKT and ERK signaling pathways. These findings indicate that p,p╩╣-DDT, as an EDC, can modify ER expression and thereby contribute to the apoptosis of human ESCs. Furthermore, when ESCs were treated with the ER inhibitor ICI, reversals were observed in ROS production and antioxidant gene expression. This suggests that the apoptosis induced by p,p╩╣-DDT exposure is related to changes in ERs, proposing a potential mechanism for p,p╩╣-DDT-induced apoptosis in human ESCs.
NF-╬║B is an important ROS-sensitive transcription regulator that participates in the cell signaling pathway alongside I╬║B [38]. Under certain stress conditions, such as exposure to environmental toxins, NF-╬║B can shift the balance between proapoptotic and antiapoptotic signals. I╬║B facilitates the transcriptional activity of NF-╬║B by undergoing phosphorylation and subsequent degradation [28,39]. Our study revealed an increase in NF-╬║B levels concurrent with a decrease in I╬║B, as well as changes in the MAPK/ERK and PI3K/AKT pathways following exposure to p,p╩╣-DDT. These findings suggest that these pathways may be involved in the mechanisms by which p,p╩╣-DDT induces apoptosis and oxidative stress. Similar to the proapoptotic role of ERK [40], the exact regulatory mechanisms of the PI3K/AKT pathway that specifically reverse cell survival, restrict growth, and promote apoptosis in ESCs warrant further detailed investigation.
This study demonstrated that p,p╩╣-DDT induces an imbalance between cell proliferation and apoptosis in human ESCs by inducing oxidative stress, altering ER expression, and impacting the PI3K-AKT/ERK/NF-╬║B signaling pathways. These findings indicate that the disruption of apoptosis triggered by p,p╩╣-DDT may play a critical role in the onset of diseases associated with the endometrium through various intracellular processes. To the best of our knowledge, this is the inaugural study to assess the impact of p,p╩╣-DDT exposure on human ESCs and to identify its molecular mediators. Further research focused on specific diseases is necessary to clarify both the mechanisms and the effects of p,p╩╣-DDT on human ESCs.
Supplementary material
Supplementary material can be found via https://doi.org/10.5653/cerm.2022.05792.
Figure┬Ā1.
Effects of p,p╩╣-bis-[4-chlorophenyl]-1,1,1-trichloroethane (DDT) on the apoptosis of human endometrial stromal cells (ESCs). (A) ESCs were treated with p,p╩╣-DDT at concentrations of 0, 1,000, and 1,000 pg/mL+fulvestrant (ICI 182,780) for 72 hours, after which apoptosis was assessed using flow cytometry. The flow cytometric analysis involved the determination of levels of propidium iodide (PI) and annexin V-fluorescein isothiocyanate (FITC). Cells that were negative for both PI and annexin V-FITC, indicating normal viable cells, are depicted in the lower left quadrant (D3). The upper left quadrant (D1) displays cells that were positive for PI but negative for annexin V-FITC, suggesting necrotic cells. Cells that were positive for annexin V-FITC and negative for PI, indicative of early apoptosis, are shown in the lower right quadrant (D4). Finally, the upper right quadrant (D2) contains cells that were positive for both PI and annexin V-FITC, denoting late (end-stage) apoptotic cells. (B) Histogram illustrating the percentages of necrotic (dead), early apoptotic, and late apoptotic cells at various concentrations of p,p╩╣-DDT (0, 10, 100, and 1,000 pg/mL, along with 1,000 pg/mL+ICI). a)p<0.05; b)p<0.005; c)p<0.0005; d)p<0.0001.

Figure┬Ā2.
Effects of p,p╩╣-bis-[4-chlorophenyl]-1,1,1-trichloroethane (DDT) on apoptotic pathway signaling in human endometrial stromal cells (ESCs). (A, B) ESCs were treated with p,p╩╣-DDT at concentrations of 0, 1,000, and 1,000 pg/mL+fulvestrant (ICI) for 72 hours. Using quantitative reverse transcription-polymerase chain reaction, we assessed the messenger RNA expression levels of genes involved in the intrinsic and extrinsic apoptotic pathways, including B-cell lymphoma-extra large (Bcl-XL), B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax), caspase-3, caspase-6, and caspase-8, in the ESCs. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as an internal control for the experiments. The reported values are the means derived from three separate experiments, with the data normalized accordingly. (C) Morphological evidence of apoptosis induced by p,p╩╣-DDT. 4ŌĆ▓,6-Diamidino-2-phenylindole (DAPI)-stained micrographs depict control (left) and DDT-exposed (right) ESCs. These images were obtained using cytometric analysis and fluorescence microscopy. (D) Immunoblots for (a) Bax and Bcl-2 and (b) FAS, caspase-3, and caspase-9. The cells were exposed to various concentrations of p,p╩╣-DDT for 72 hours, followed by analysis through Western blotting. Protein levels were normalized to ╬▓-actin (the loading control) and are presented as relative fold changes in comparison to control values. Data are expressed as the mean┬▒standard error of the mean. a)p-values that denote statistical significance when compared to the control cells at the corresponding time point, as determined by the Student t-test (p<0.05); b)p<0.005 c)p-values that denote statistical significance when compared to the control cells at the corresponding time point, as determined by analysis of variance (p<0.05).

Figure┬Ā3.
Effects of p,p╩╣-bis-[4-chlorophenyl]-1,1,1-trichloroethane (DDT) exposure on oxidative stress in human endometrial stromal cells. (A) Representative flow cytometry measurements of basal reactive oxygen species (ROS) generation were conducted using 2,7-dichlorofluorescein diacetate (DCF-DA). ROS production was assessed via fluorescence-activated cell sorting following 72 hours of treatment with DDT with or without fulvestrant (ICI). The data are presented as the mean┬▒standard error of the mean, derived from a minimum of three independent experiments. The mean fluorescence intensity of the DCF-DA-stained cells was analyzed. (B) Confocal images depicting changes in ROS production following exposure to p,p╩╣-DDT at a concentration of 1,000 pg/mL, as well as after ICI treatment. Variations in ROS levels are evidenced by the DCF-DA fluorescence. All images were captured at the same magnification. (C) Quantitative reverse transcription-polymerase chain reaction analysis of antioxidant enzymes. The messenger RNA (mRNA) expression levels of glutathione peroxidase (GPX), superoxide dismutase (SOD), catalase (CAT), and heme oxygenase (HO) were analyzed, with all mRNA expression values normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. The data are presented as the mean┬▒standard error of the mean, derived from a minimum of three independent experiments. a)p-values that denote statistical significance when compared to the control cells at the corresponding time point, as determined by the Student t-test (p<0.05); b)p<0.005; c)p<0.0005.
Figure┬Ā4.
Effects of p,p╩╣-bis-[4-chlorophenyl]-1,1,1-trichloroethane (DDT) on estrogen receptor (ER) expression in human endometrial stromal cells (ESCs). (A) After exposure to p,p╩╣-DDT at concentrations of 10, 100, 1,000, or 1,000 pg/mL with fulvestrant (ICI), ESCs were subjected to reverse transcription-polymerase chain reaction analysis to quantify the messenger RNA expression levels of ER-╬▒ and ER-╬▓. Expression values were normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a loading control and are presented as relative fold changes in comparison to control values. Data are expressed as the mean┬▒standard error of the mean (SEM). (B) Immunoblots of ER-╬▒ and ER-╬▓. Cells were treated with p,p╩╣-DDT for 72 hours and analyzed using western blotting. All protein levels were normalized to the level of ╬▓-actin (the loading control) and presented as relative fold changes in comparison to control values. The results are shown as the mean┬▒SEM of the data. a)p-values that denote statistical significance relative to the control cells at the corresponding time point, as determined by the Student t-test (p<0.05); b)p-values that denote statistical significance when compared to the control cells at the corresponding time point, as determined by analysis of variance (p<0.05); c)p<0.0005.
Figure┬Ā5.
Effects of p,p╩╣-bis-[4-chlorophenyl]-1,1,1-trichloroethane (DDT) on the protein kinase B (AKT) signaling pathway and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-╬║B)/I╬║B expression levels. The study investigated the effects of p,p╩╣-DDT exposure on the AKT and extracellular signal-regulated kinase (ERK) signaling pathways (A, B, C) and on the levels of NF-╬║B and I╬║B proteins (D, E). After treatment with p,p╩╣-DDT (at 10, 100, 1,000, or 1,000 pg/mL+fulvestrant [ICI]), the cells were harvested for analysis. Western blotting was employed to measure the protein levels of phosphatidylinositol 3-kinase (PI3K), phospho-AKT, phospho-ERK, NF-╬║B, and I╬║B. ╬▓-Actin served as the loading control for these assays, and the protein levels were normalized to ╬▓-actin for quantitative analysis. The measurements are presented as relative fold changes in comparison to the control cells. These results represent the mean┬▒standard error of the mean, based on data from three independent experiments. a)p-values that denote statistical significance when compared to the control cells at the corresponding time point, as determined by the Student t-test (p<0.05); b)p<0.005.
Figure┬Ā6.
Intrinsic and extrinsic apoptotic pathways in human endometrial stromal cells induced by p,p╩╣-bis-[4-chlorophenyl]-1,1,1-trichloroethane (DDT). In this model, we propose that p,p╩╣-DDT promotes the induction of cytochrome C by elevating expression of Bcl-2-associated X protein (Bax) and decreasing expression of B-cell lymphoma 2 (Bcl-2). This alteration in Bax and Bcl-2 expression then acts in conjunction with apoptotic protease activating factor 1 (APAF-1) to activate procaspase 9, which in turn triggers the extrinsic pathway, leading to apoptosis. Mito, mitochondria.

References
1. Ferenczy A, Bertrand G, Gelfand MM. Proliferation kinetics of human endometrium during the normal menstrual cycle. Am J Obstet Gynecol 1979;133:859-67.


2. Guzeloglu Kayisli O, Kayisli UA, Luleci G, Arici A. In vivo and in vitro regulation of Akt activation in human endometrial cells is estrogen dependent. Biol Reprod 2004;71:714-21.


3. Bartelmez GW. Premenstrual and menstrual ischemia and the myth of endometrial arteriovenous anastomoses. Am J Anat 1956;98:69-95.


4. Fanger H, Barker BE. Capillaries and arterioles in normal endometrium. Obstet Gynecol 1961;17:543-50.

5. Hopwood D, Levison DA. Atrophy and apoptosis in the cyclical human endometrium. J Pathol 1976;119:159-66.


6. Kokawa K, Shikone T, Nakano R. Apoptosis in the human uterine endometrium during the menstrual cycle. J Clin Endocrinol Metab 1996;81:4144-7.


7. Beard J, Australian Rural Health Research Collaboration. DDT and human health. Sci Total Environ 2006;355:78-89.


8. Cho YJ, Yun JH, Kim SJ, Kwon HY. Nonpersistent endocrine disrupting chemicals and reproductive health of women. Obstet Gynecol Sci 2020;63:1-12.




9. Piazza MJ, Urbanetz AA. Environmental toxins and the impact of other endocrine disrupting chemicals in womenŌĆÖs reproductive health. JBRA Assist Reprod 2019;23:154-64.



10. Turusov V, Rakitsky V, Tomatis L. Dichlorodiphenyltrichloroethane (DDT): ubiquity, persistence, and risks. Environ Health Perspect 2002;110:125-8.



11. Smith AG. DDT and its analogs. In: Krieger RI, Krieger WC, editors. Handbook of pesticide toxicology. 2nd ed. Elsevier; 2001. p. 1305-55.
12. Cho YJ, Park SB, Han M. Di-(2-ethylhexyl)-phthalate induces oxidative stress in human endometrial stromal cells in vitro. Mol Cell Endocrinol 2015;407:9-17.


13. Cho YJ, Park SB, Park JW, Oh SR, Han M. Bisphenol A modulates inflammation and proliferation pathway in human endometrial stromal cells by inducing oxidative stress. Reprod Toxicol 2018;81:41-9.


14. Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. EDC-2: The Endocrine SocietyŌĆÖs second scientific statement on endocrine-disrupting chemicals. Endocr Rev 2015;36:E1-150.



15. Kojima H, Katsura E, Takeuchi S, Niiyama K, Kobayashi K. Screening for estrogen and androgen receptor activities in 200 pesticides by in vitro reporter gene assays using Chinese hamster ovary cells. Environ Health Perspect 2004;112:524-31.



16. Rogan WJ, Gladen BC, McKinney JD, Carreras N, Hardy P, Thullen J, et al. Polychlorinated biphenyls (PCBs) and dichlorodiphenyl dichloroethane (DDE) in human milk: effects of maternal factors and previous lactation. Am J Public Health 1986;76:172-7.



17. Saxena MC, Siddiqui MK, Seth TD, Krishna Murti CR, Bhargava AK, Kutty D. Organochlorine pesticides in specimens from women undergoing spontaneous abortion, premature of full-term delivery. J Anal Toxicol 1981;5:6-9.


18. Windham GC, Lee D, Mitchell P, Anderson M, Petreas M, Lasley B. Exposure to organochlorine compounds and effects on ovarian function. Epidemiology 2005;16:182-90.


19. van Wendel de Joode B, Wesseling C, Kromhout H, Monge P, Garcia M, Mergler D. Chronic nervous-system effects of long-term occupational exposure to DDT. Lancet 2001;357:1014-6.


20. Cooper GS, Savitz DA, Millikan R, Chiu Kit T. Organochlorine exposure and age at natural menopause. Epidemiology 2002;13:729-33.


21. Venners SA, Korrick S, Xu X, Chen C, Guang W, Huang A, et al. Preconception serum DDT and pregnancy loss: a prospective study using a biomarker of pregnancy. Am J Epidemiol 2005;162:709-16.


23. Schwartzman RA, Cidlowski JA. Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocr Rev 1993;14:133-51.


25. Bredhult C, Backlin BM, Olovsson M. Effects of some endocrine disruptors on the proliferation and viability of human endometrial endothelial cells in vitro. Reprod Toxicol 2007;23:550-9.


26. Bredhult C, Sahlin L, Olovsson M. Gene expression analysis of human endometrial endothelial cells exposed to opŌĆÖ-DDT. Mol Hum Reprod 2008;14:97-106.


27. Holloway AC, Stys KA, Foster WG. DDE-induced changes in aromatase activity in endometrial stromal cells in culture. Endocrine 2005;27:45-50.


28. Frigo DE, Burow ME, Mitchell KA, Chiang TC, McLachlan JA. DDT and its metabolites alter gene expression in human uterine cell lines through estrogen receptor-independent mechanisms. Environ Health Perspect 2002;110:1239-45.



29. Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998;139:4252-63.


30. Snezhkina AV, Kudryavtseva AV, Kardymon OL, Savvateeva MV, Melnikova NV, Krasnov GS, et al. ROS generation and antioxidant defense systems in normal and malignant cells. Oxid Med Cell Longev 2019;2019:6175804.




31. Pang W, Zhang Y, Zhao N, Darwiche SS, Fu X, Xiang W. Low expression of Mfn2 is associated with mitochondrial damage and apoptosis in the placental villi of early unexplained miscarriage. Placenta 2013;34:613-8.


32. Marouani N, Hallegue D, Sakly M, Benkhalifa M, Ben Rhouma K, Tebourbi O. p,pŌĆÖ-DDT induces testicular oxidative stress-induced apoptosis in adult rats. Reprod Biol Endocrinol 2017;15:40.




33. Perez-Maldonado IN, Diaz-Barriga F, de la Fuente H, Gonzalez-Amaro R, Calderon J, Yanez L. DDT induces apoptosis in human mononuclear cells in vitro and is associated with increased apoptosis in exposed children. Environ Res 2004;94:38-46.


34. Lee HR, Kim TH, Choi KC. Functions and physiological roles of two types of estrogen receptors, ER╬▒ and ER╬▓, identified by estrogen receptor knockout mouse. Lab Anim Res 2012;28:71-6.



35. Hewitt SC, Harrell JC, Korach KS. Lessons in estrogen biology from knockout and transgenic animals. Annu Rev Physiol 2005;67:285-308.


36. Uchida S, Saimi M, Li ZL, Miyaso H, Nagahori K, Kawata S, et al. Effects of phosphorylated estrogen receptor alpha on apoptosis in human endometrial epithelial cells. Anat Sci Int 2020;95:240-50.



37. Salvesen HB, Carter SL, Mannelqvist M, Dutt A, Getz G, Stefansson IM, et al. Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proc Natl Acad Sci U S A 2009;106:4834-9.



38. Mulero MC, Huxford T, Ghosh G. NF-╬║B, I╬║B, and IKK: Integral Components of Immune System Signaling. Adv Exp Med Biol 2019;1172:207-26.


- TOOLS







