 |
 |
- Search
| Clin Exp Reprod Med > Volume 51(2); 2024 > Article |
|
Abstract
Objective
Seasonal variations in semen quality are known to occur in temperate regions, but results regarding tropical areas remain inconclusive. The aim of this study was to determine whether monthly variations in semen parameters are present among men in a tropical region.
Methods
Data were retrospectively collected from semen analyses of 3,000 men over a 10-year period, from 2012 to 2022. Analysis of variance and the independent-samples t-test were employed to observe variations in semen parameters throughout the entire period and between months, respectively.
Results
The mean±standard deviation sperm concentration was significantly lower in June, at 42.5±31.4 million/mL, compared to other months. The highest sperm concentration was found in March, at 57.8±42.6 million/mL, constituting a mean difference of 15.3 million/mL between the lowest and highest concentrations. The total sperm count displayed a similar pattern of monthly variation, with a difference of 47.2 million between the highest and lowest months. No significant monthly differences were observed in other parameters, such as sperm motility, morphology, and semen volume.
Conclusion
Significant monthly variations in sperm concentration and total sperm count were evident in this Sri Lankan population. March, which displayed the highest sperm counts, is in the spring in temperate regions, while the month with the lowest counts, July, is part of the summer. Fluctuations in photoperiod appear to most strongly influence these variations.
Temporary or permanent deterioration of semen quality is a prominent factor contributing to reduced fertility potential in men. Seasonality-induced partial and reversible changes in semen characteristics are well-documented in numerous animal species, including bulls, boars, goats, rams, and monkeys [1-5]. This phenomenon has been recognized since the 1940s [6], with a broad range of results observed in various studies conducted across the world. However, no consensus has been reached regarding the most or least favorable season for semen quality, or regarding the extent of variation in individual semen parameters during specific seasons. The inconsistency in study results can be attributed to factors such as regional variations in periodicity, individual differences in semen quality, and discrepancies in sample size, study design, and the animal species used.
Possible causes of fluctuating semen quality include the resetting of the endogenous biological clock and subsequent changes in the anatomy and physiology of the male reproductive system in response to variations in photoperiod and temperature [5,7]. Reports have also noted changes in other biochemical factors that precede or co-occur with alterations in semen quality in animals. These factors include seminal plasma protein level; antiperoxidant activity [8]; testosterone, luteinizing hormone, and non-esterified fatty acid levels [9]; lipid peroxidation; the production of reactive oxygen species [10]; and melatonin level [11].
In humans, as in most other species, reproductive functions and fecundability are influenced by seasonal and diurnal rhythms. An increase in sperm count and motility has been observed in afternoon collections compared to morning samples, although the underlying mechanism remains unclear [12]. In general, studies conducted in temperate climates have demonstrated chronobiological fluctuations in various semen parameters, with relatively poor semen quality observed during the summer or fall and an increase noted in winter and spring [13,14]. The variations in sperm number or function may be due in part to changes in the endocrine profile, such as levels of melatonin, follicle-stimulating hormone, inhibin B, estradiol, and testosterone [15]. Research has suggested that lower sperm quality in the summer may impact natural conception in women, potentially contributing to observed decreases in births in the spring [16]. However, the consistency of these results may be influenced by confounding factors such as age, days of abstinence, and smoking, among others [13,14,17].
The question at hand is whether sperm count or functionality fluctuates with minor or short-term variations in temperature and day length, as seen in tropical regions. In one prior study, Chia et al. [18] observed no monthly variations in semen quality. However, Kunzle et al. [13] noted a decline in sperm counts and motility during the warmer season among smokers with borderline fertility. The data from the present study could be instrumental in settling the ongoing debate.
We retrieved semen analysis records from men who had attended fertility evaluations for data collection between 2012 and 2022. The inclusion criterion for samples was a sperm concentration exceeding 5 million/mL. We excluded severely oligozoospermic samples due to the multitude of potential causes, in an effort to minimize any influence on the accuracy of the results. The first 25 semen analysis records that met the selection criteria each month were included in the analysis.
The laboratory adhered to the standard protocols for semen analysis as outlined in the 2010 World Health Organization guidelines [19]. Samples were gathered following 3 to 5 days of ejaculatory abstinence, and each sample was analyzed by one of the two experienced technicians available at the time. Variations between technicians were periodically evaluated as part of the laboratory’s quality control program. Semen volume, sperm concentration, motility, and morphology were compared across different months of the year via analysis of variance and the individual-samples t-test, using SPSS ver. 16.0 (SPSS Inc.).
This study was approved by the Ethics Review Committee of the Faculty of Medicine, University of Kelaniya (P/34/04/2021). Written informed consent by the patients was waived due to a retrospective nature of our study.
The age of the study population, expressed as the mean±standard deviation, was 33±5.9 years (range, 19 to 55). The mean semen volume was 2.6±1.6 mL (range, 0.5 to 10.0). The mean values for sperm concentration, total count, motility, and morphology were as follows: 50.8±35.5 million/mL (range, 2.2 to 293.5), 129.6±107.9 million (range, 1.9 to 816.0), 53%±18% (range, 0% to 96%), and 43%±17.8% (range, 0% to 68%), respectively.
Table 1 provides a decade-long overview of annual variations in semen parameters. Between years, significant fluctuations were observed in the mean values of sperm concentration and total count. Both parameters peaked in 2017, with the lowest values recorded in 2020 and 2012, respectively. The variations in age, mean volume, and morphology remained within a narrow range, with no significant trends emerging over the 10-year period.
Correlation analysis revealed negative associations between age and both motility (r=0.56, p=0.01) and morphology (r=0.045, p=0.038), but no such relationship was found with the other semen parameters. The monthly variations of these parameters are detailed in Table 2. No consistent pattern of variations was observed in morphology or motility across months. However, semen volume, sperm count, and total count displayed a remarkably consistent pattern of monthly variation, with high values observed in March and April and low values in June and July (Figure 1). Pronounced mean differences were found between the months with the highest and lowest values of these parameters. Specifically, the differences found were 0.26 mL for volume, 15.3 million/mL for concentration, and 47.2 million for total sperm count.
Figure 1 illustrates the consistent pattern of variation in semen parameters. Sperm concentration and total count exhibited similar patterns, displaying decreased values during months with longer daylight hours and increased values in months with relatively cold temperatures and shorter daylight hours. While semen volume also followed this pattern, those variations were not statistically significant.
As a tropical country, Sri Lanka experiences only minor chronobiological variations in temperature and photoperiod compared to temperate regions. Meteorological data indicate that the warmest months are April and May, while the coldest extend from November to February, with a difference of 2.4 °C. The longest days occur in June, with approximately 45 more minutes of daylight than the shortest, which take place in December. These data pertain to the western province in which our lab is located, and minor regional variations have been recorded in the central and dry zones of the country. However, our center receives referrals from other regions in addition to the western province. Therefore, we believe that the results obtained accurately represent the effects of real climatic changes in this tropical country.
Our results indicated significant monthly fluctuations in sperm concentration and total sperm count, with the lowest values observed in June and the highest in March. These seminal variations appear to correlate more closely with the photoperiod than with the minor temperature fluctuations that occur throughout the year. Substantial temperature variations, coupled with changes in the photoperiod, have been postulated to cause decreased sperm production in temperate countries. However, in tropical regions, where temperature variations are minimal, spermatogenesis seems to be influenced solely by the photoperiod. The most plausible explanation for this finding is the suppression of melatonin production as the photoperiod increases. The significant impact of light on semen was clearly demonstrated by Cagnacci et al. [12], who observed samples with superior sperm count and motility in afternoon collections compared to morning samples. However, these short-term variations could be attributed to functional changes in nerve-muscle coordination during the ejaculatory process, and they may also be influenced by components of the seminal plasma [12].
In our analysis by year (data not shown), we did not observe significant fluctuations in sperm count during certain years. This could be due to the specific population selected for the study, including individual factors such as days of abstinence and age. Furthermore, for men who already have compromised semen quality for various anatomical or physiological reasons, environmental factors such as temperature, light, and lifestyle can further negatively impact spermatogenesis [13]. Politoff et al. [20] noted that during the summer months, a population with oligozoospermia exhibited a more pronounced decrease in sperm count than a group with normal counts.
Chen et al. [17] reported that sperm count, motility, and morphology were increased in the spring, while another study indicated elevated sperm count and morphology [18]. Seasonal variations have also been reported regarding sperm immaturity and tapered heads [14]. Yogev et al. [21] detected a high count of cryopreservable sperm in the winter and spring. In the present study, monthly variations were observed only in sperm concentration and total count. Our results align with most studies conducted in temperate regions; the highest sperm count was observed in March and April, coinciding with spring, while the lowest count was noted in June, corresponding with summer. The decrease in sperm count in June can be explained by an increase in the photoperiod. However, the observation of a high sperm count in March, when December has the fewest hours of daylight in this region, has sparked debate. Nevertheless, no significant difference was observed in sperm count between March and December. Such fluctuations can be anticipated, as our study population comprised both normozoospermic and pathozoospermic samples. The unequal distribution of subnormal samples throughout the year may have slightly skewed the results. Moreover, semen parameters are highly variable even within a given individual, and using a single sample to assess quality may introduce errors.
As with some prior research, we identified no significant chronobiological variations in the other semen parameters. Greater motility in winter samples has been described as being due to an increased secretion of melatonin [15]. The potential effect of melatonin on the circannual variations of sperm nuclear maturity was suggested by Henkel et al. [22]. However, we did not observe a significant effect of temperature on seminal variations. Zhang et al. [23] suggested that seasonal fluctuations in semen parameters are primarily due to temperature variations, with photoperiod having only a minor influence. However, the variation in temperature in their study region was high, at more than 10 °C, compared to the minor variations in our region [23]. It is well-known that significant impacts on spermatogenesis tend to manifest around 3 months after the inciting event. The mechanism behind the lowering of sperm count in the same month as the highest photoperiod in our study is unclear. Endogenous factors have also been suggested to play a key role in sperm count fluctuations [20].
In conclusion, despite the lack of distinct seasonality in the tropics, sperm production appears to be influenced by minor variations in environmental factors, particularly the duration of the photoperiod. A decrease in sperm count due to changes in photoperiod could have significant implications for men with severe oligozoospermia. Considerable fluctuations in semen quality may contribute to the poor outcomes observed in assisted reproduction. Further research is required to substantiate this hypothesis.
Figure 1.
Monthly variations in semen parameters (n=3,000). Along the x-axis, month 1 indicates January, 2 corresponds to February, etc.
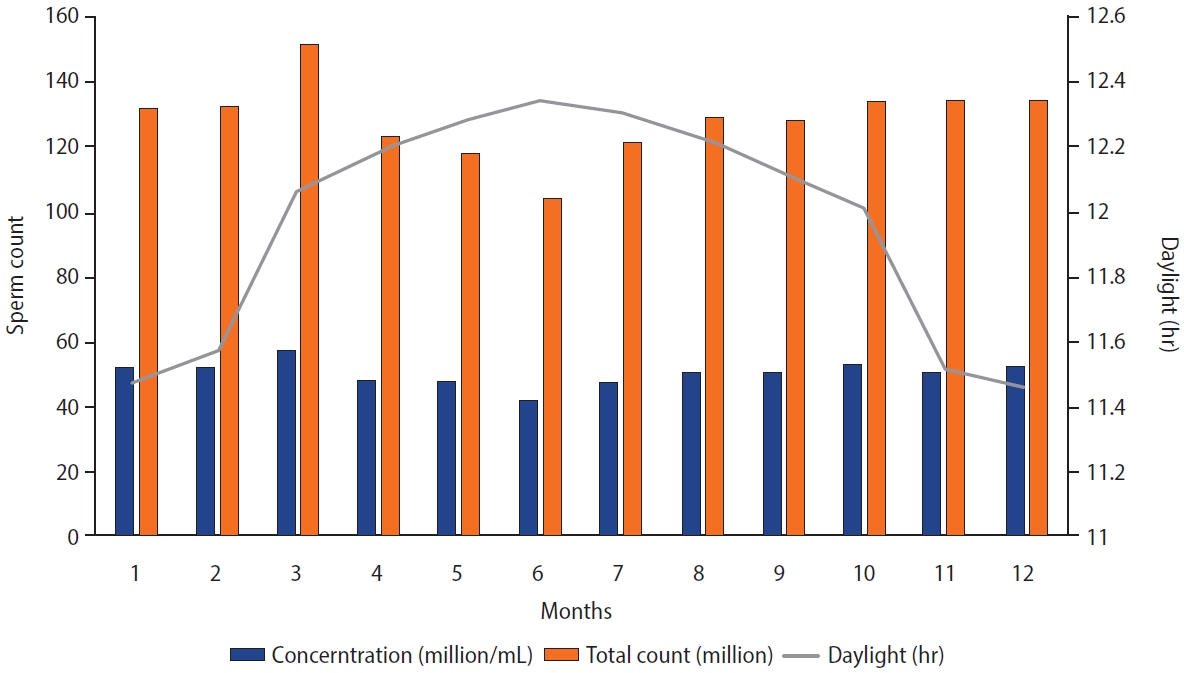
Table 1.
Annual variations in semen parameters over 10 years (n=3,000a))
Table 2.
Monthly variations in semen parameters (n=3,000a))
References
1. Valeanu S, Johannisson A, Lundeheim N, Morrell JM. Seasonal variation in sperm quality parameters in Swedish red dairy bulls used for artificial insemination. Livest Sci 2015;173:111-8.

2. Petrocelli H, Batista C, Gosalvez J. Seasonal variation in sperm characteristics of boars in southern Uruguay. Rev Bras Zootec 2015;44:1-7.

3. Kridli RT, Tabbaa MJ, Barakeh FS. Seasonal variation in scrotal circumference and semen characteristics of Black Bedouin and Black Bedouin-Damascus crossbred bucks. Asian-Australas J Anim Sci 2007;20:359-64.

4. Karagiannidis A, Varsakeli S, Alexopoulos C, Amarantidis II. Seasonal variation in semen characteristics of Chios and Friesian rams in Greece. Small Rumin Res 2000;37:125-30.


5. Zamboni L, Conaway CH, Van Pelt L. Seasonal changes in production of semen in free-ranging rhesus monkey. Biol Reprod 1974;11:251-67.


6. Erb RE, Andrews FN, Hilton JH. Seasonal variation in semen quality of the dairy bull. J Dairy Sci 1942;25:815-26.

7. Levine RJ. Seasonal variation of semen quality and fertility. Scand J Work Environ Health 1999;25 Suppl 1:34-7.

8. Zasiadczyk L, Fraser L, Kordan W, Wasilewska K. Individual and seasonal variations in the quality of fractionated boar ejaculates. Theriogenology 2015;83:1287-303.


9. Parkinson TJ. Seasonal variation in semen quality of bulls and correlations with metabolic and endocrine parameters. Vet Rec 1985;117:303-7.


10. Alam SS, El Makawy AI, Tohamy AA, Abd Elrahman MM. Effect of seasonal variations on semen quality and fertility of Egyptian water buffalo (Bubalus bubalis) bulls. Res J Pharm Biol Chem Sci 2015;6:1059-69.
11. Casao A, Cebrian I, Asumpcao ME, Perez-Pe R, Abecia JA, Forcada F, et al. Seasonal variations of melatonin in ram seminal plasma are correlated to those of testosterone and antioxidant enzymes. Reprod Biol Endocrinol 2010;8:59.



12. Cagnacci A, Maxia N, Volpe A. Diurnal variation of semen quality in human males. Hum Reprod 1999;14:106-9.


13. Kunzle R, Mueller MD, Huber AW, Drescher H, Bersinger NA. Seasonality in human semen quality of smokers and non-smokers: effect of temperature. Asian J Androl 2004;6:243-7.

14. Chen Z, Toth T, Godfrey-Bailey L, Mercedat N, Schiff I, Hauser R. Seasonal variation and age-related changes in human semen parameters. J Androl 2003;24:226-31.


15. De Giorgi A, Volpi R, Tiseo R, Pala M, Manfredini R, Fabbian F. Seasonal variation of human semen parameters: a retrospective study in Italy. Chronobiol Int 2015;32:711-6.


16. Levitas E, Lunenfeld E, Weisz N, Friger M, Har-Vardi I. Seasonal variations of human sperm cells among 6455 semen samples: a plausible explanation of a seasonal birth pattern. Am J Obstet Gynecol 2013;208:406.

17. Chen Z, Godfrey-Bailey L, Schiff I, Hauser R. Impact of seasonal variation, age and smoking status on human semen parameters: The Massachusetts General Hospital experience. J Exp Clin Assist Reprod 2004;1:2.




18. Chia SE, Lim ST, Ho LM, Tay SK. Monthly variation in human semen quality in male partners of infertile women in the tropics. Hum Reprod 2001;16:277-81.


19. World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. WHO Press; 2010.
20. Politoff L, Birkhauser M, Almendral A, Zorn A. New data confirming a circannual rhythm in spermatogenesis. Fertil Steril 1989;52:486-9.


21. Yogev L, Kleiman S, Shabtai E, Botchan A, Gamzu R, Paz G, et al. Seasonal variations in pre- and post-thaw donor sperm quality. Hum Reprod 2004;19:880-5.









