|
 |
- Search
| Clin Exp Reprod Med > Volume 51(1); 2024 > Article |
|
Abstract
Objective
This study evaluated the effects of temperature and storage time on the quality and DNA integrity of freeze-dried sperm from individuals with normozoospermia.
Methods
Normal sperm samples from 15 men aged 24 to 40 years were studied. Each sample was divided into six groups: fresh, freezing (frozen in liquid nitrogen), freeze-dried then preserved at room temperature for 1 month (FD-1m-RT), freeze-dried then preserved at room temperature for 2 months (FD-2m-RT), freeze-dried then preserved at 4 ┬░C for 1 month (FD-1m-4 ┬░C), and freeze-dried then preserved at 4 ┬░C for 2 months (FD-2m-4 ┬░C). The morphology, progressive motility, vitality, and DNA integrity of the sperm were evaluated in all groups.
Results
In all freeze-dried groups, sperm cells were immotile after rehydration. The freeze-dried groups also showed significantly less sperm vitality than the fresh and frozen groups. Significantly more morphological sperm abnormalities were found in the freeze-dried groups, but freeze-drying did not lead to a significantly higher DNA fragmentation index (DFI). The DFI was significantly higher in the FD-2m-RT group than in the other freeze-dried groups.
Protective cryopreservation of sperm is performed routinely at many assisted reproductive centers today. Although this method is widely used, the process of freezing and thawing affects sperm function and DNA integrity [1]. In fact, only 50% of sperm survive after the freezing and thawing process [1-3]. This method also damages the DNA of sperm cells [4] and thus has a negative effect on the results of fertilization and embryo quality [5].
Cryopreservation is the most widely used method for preserving human and other animal sperm. In this method, liquid nitrogen (LN2) is needed to store sperm for a long time. In recent years, there has been interest in developing alternative techniques for storing and transporting sperm. The freeze-drying method for sperm is a cheaper and healthier method, especially since there is no need for antifreeze and LN2. Another advantage is that freeze-dried sperm can be stored at room temperature and do not require a special storage container [6]. Producing live offspring from freeze-dried sperm was first demonstrated 24 years ago [7]. Since then, the use of freeze-dried sperm has been reported for a number of species, including rats, mice, cats, dogs, rabbits, bulls, pigs, horses, chimpanzees, giraffes, jaguars, minks, and humans [8].
Lyophilization, freeze-drying, is a protective method in which frozen material is dried by ice sublimation. The main purpose of freeze-drying is to move water out of the system to stop chemical reactions and biological growth. During this process, by sublimation (initial drying) and then by repelling (the second stage of drying), the base material is frozen and then the amount of solvent is reduced [9]. Freeze-drying was first used 60 years ago for rooster sperm cells [10]. The first successful live birth with this method was reported in 1998, with the intracytoplasmic sperm injection (ICSI) technique for mouse sperm [7,11].
As a method of genetic storage, freeze-dried sperm can serve as a good alternative to the routine protective cryopreservation of sperm. Since sperm cells lose their motility and vitality after freeze-drying, ICSI should be used upon fertilization. Such sperm can lead to the birth of live offspring [6]. Furthermore, because studies have shown that freeze-drying causes less damage to sperm DNA than conventional freezing, a more detailed investigation of this method would provide important insights on how to improve the quality of freeze-dried sperm. Therefore, this study investigated the effect of temperature and storage time on the quality and DNA integrity of freeze-dried sperm.
The study was approved by the Research Ethics Committee of the Iran University of Medical Sciences (research project code 98-4-4-15555) (IR.IUMS.FMD.REC.1399.089), and all participants provided informed consent.
The semen samples were taken from 15 men aged 24 to 40 who had been referred to the Akbarabadi IVF Clinic. Samples from patients with a history of disease (such as varicocele, cryptorchidism, or systemic diseases) were excluded from the study. All participants had 3 to 5 days of sexual abstinence prior to sampling. After liquefaction, the samples were analyzed, and those that had normal parameters according to the World Health Organization criteria were included in the study (mean┬▒standard deviation [SD] of sperm parameters: count, 80.80┬▒28.15 million; morphology, 6.26┬▒2.96%; motility, 60.33┬▒8.63% vitality, 69.67┬▒10.93%) [12]. All chemicals were purchased from Sigma Aldrich unless otherwise stated.
Each sample was divided into six groups:
ŌĆā1. Fresh
ŌĆā2. Freeze: frozen in LN2
ŌĆā3. FD-1m-RT: after freeze-drying, preserved at room temperature for 1 month
ŌĆā4. FD-2m-RT: after freeze-drying, preserved at room temperature for 2 months
ŌĆā5. FD-1m-4 ┬░C: after freeze-drying, preserved at 4 ┬░C for 1 month
ŌĆā6. FD-2m-4 ┬░C: after freeze-drying, preserved at 4 ┬░C for 2 months
The samples of all freezing groups were frozen and thawed after 1 month and were examined in terms of motility, morphology, vitality, and DNA integrity.
Cryopreservation was done according to the freezing method. After the sperm freezing medium (Origio; CooperSurgical) was added to the sperm suspension in an equal volume (1:1; 0.1 mL/min), the sample was preserved at 37 ┬░C for 5 minutes. Next, the sample was exposed to LN2 vapor for 15 minutes and then plunged into LN2. One month after freezing, the sample was thawed. After thawing, to remove the cryoprotectant, the suspension was washed in T6 medium [13].
For freeze-drying, 100 ╬╝L of each sperm sample was added to a cryovial containing 400 ╬╝L of buffer solution (1 mM of ethylenediaminetetraacetic acid [EDTA] and 10 mM Tris-HCl) and frozen at ŌłÆ20 ┬░C for 6 hours. Then the cryovial was transferred to a programmable freeze-dry machine (Pishtaz Equipment Company) near the condenser and subjected to vacuum treatment overnight at 220├Ś10ŌłÆ3 mbar pressure. Immediately before evaluation, the vials of freeze-dried sperm were unsealed, and the sperm were hydrated with 500 ╬╝L sterile distilled water [13].
Progressively motile sperm were identified as moving forward in a straight line or large circles by Makler Chamber and a light microscope at ├Ś400 magnification (Motic BA410; Kowloon). Analysis was performed by examining at least 200 sperm. The technician who performed the analyses was blinded to the study.
The vital stain trypan blue was used to evaluate sperm vitality, in accordance with previous studies: 10 ╬╝L of sperm supernatant and 2 ╬╝L of formalin (10% diluted) were mixed with 10 ╬╝L of trypan blue. In dead sperm cells, trypan blue stain penetrated the postacrosomal region. At least 200 sperm were evaluated via light microscopy at ├Ś1,000 magnification. The percentage of viable, unstained sperm was then reported [14].
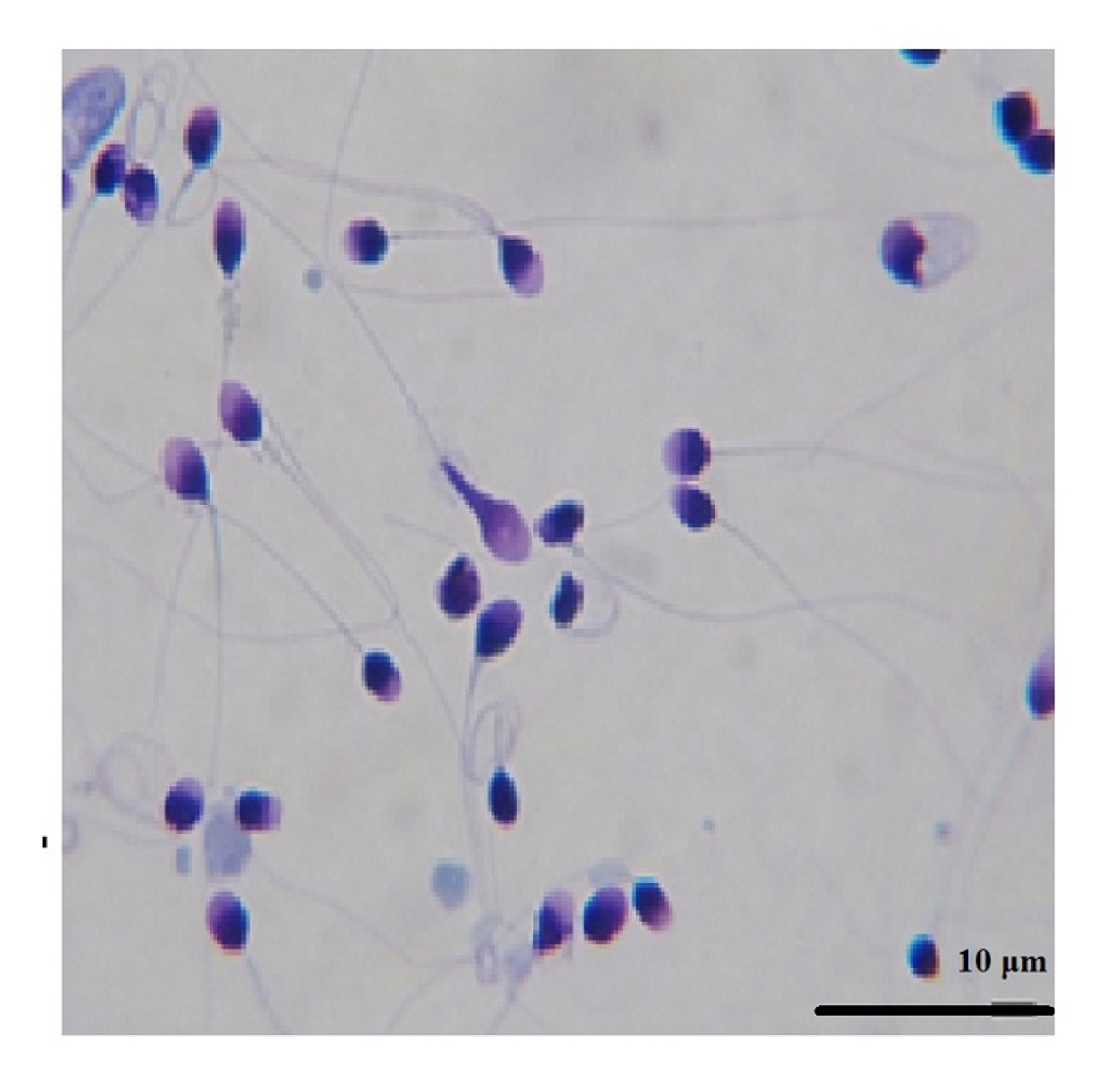
A Diff-Quik kit (BRED Life Science Technology Inc.) was used for evaluating sperm morphology. Staining was performed according to the manufacturer's instructions. At least 200 sperm were evaluated via light microscopy at ├Ś1,000 magnification. Next, the percentages of normal and abnormal sperm were reported based on Kruger's classification [13].
DNA fragmentation in the sperm samples was evaluated by the halo sperm method. The sperm concentration was adjusted to 5ŌĆÆ10 million/mL through dilution in human tubal fluid medium (Irvine Scientific). Next, the suspension was mixed with agarose, and 50 ╬╝L of it was placed on a pre-coated slide and preserved at 4 ┬░C for 4 minutes. The coverslips were then carefully removed. All solutions were used according to the manufacturer's instructions (sperm DNA fragmentation assay [SDFA] Kit, lot 18,855; ReproSource). Sperm with a large or medium halo were classified as healthy sperm without DNA fragmentation. A DNA fragmentation index (DFI) index above 30% was considered to indicate abnormal DNA integrity [15].
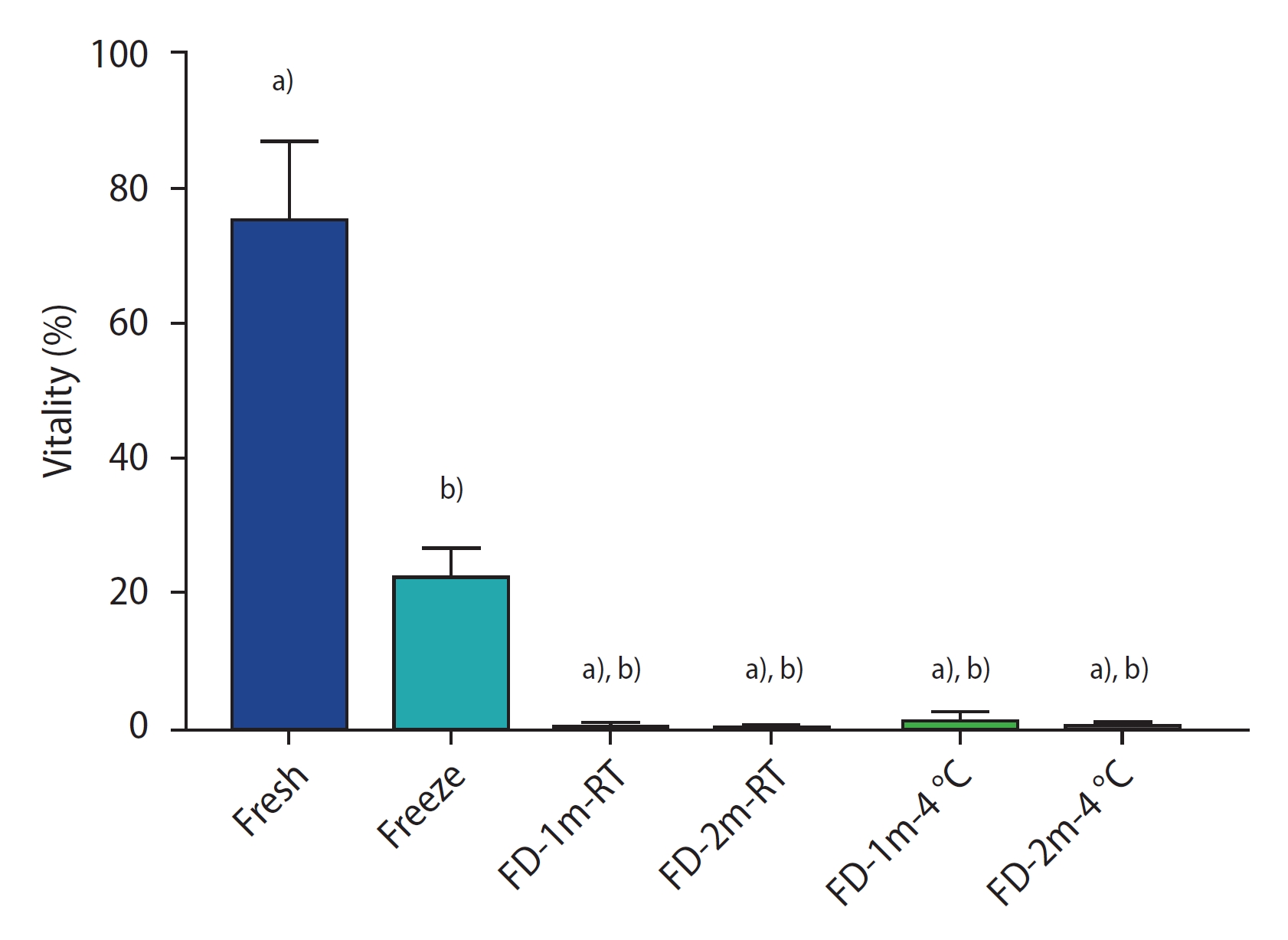
Figure 1 shows the results of progressive sperm motility in each group. In all freeze-dried groups, the sperm were immotile after rehydration. There was a significant difference between these groups and the fresh and conventional freezing groups (p<0.0001). There was no significant difference among the freeze-dried groups.
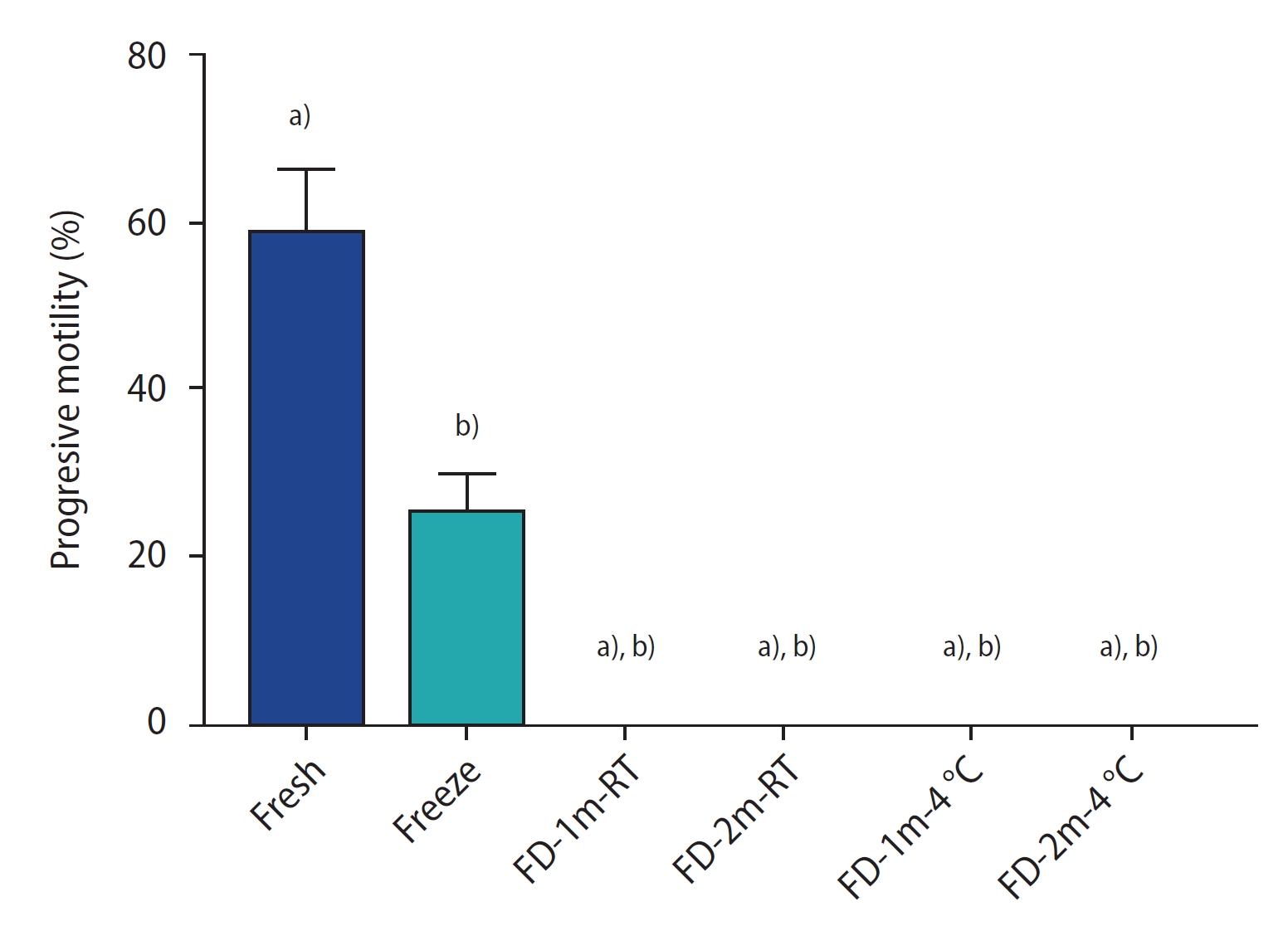
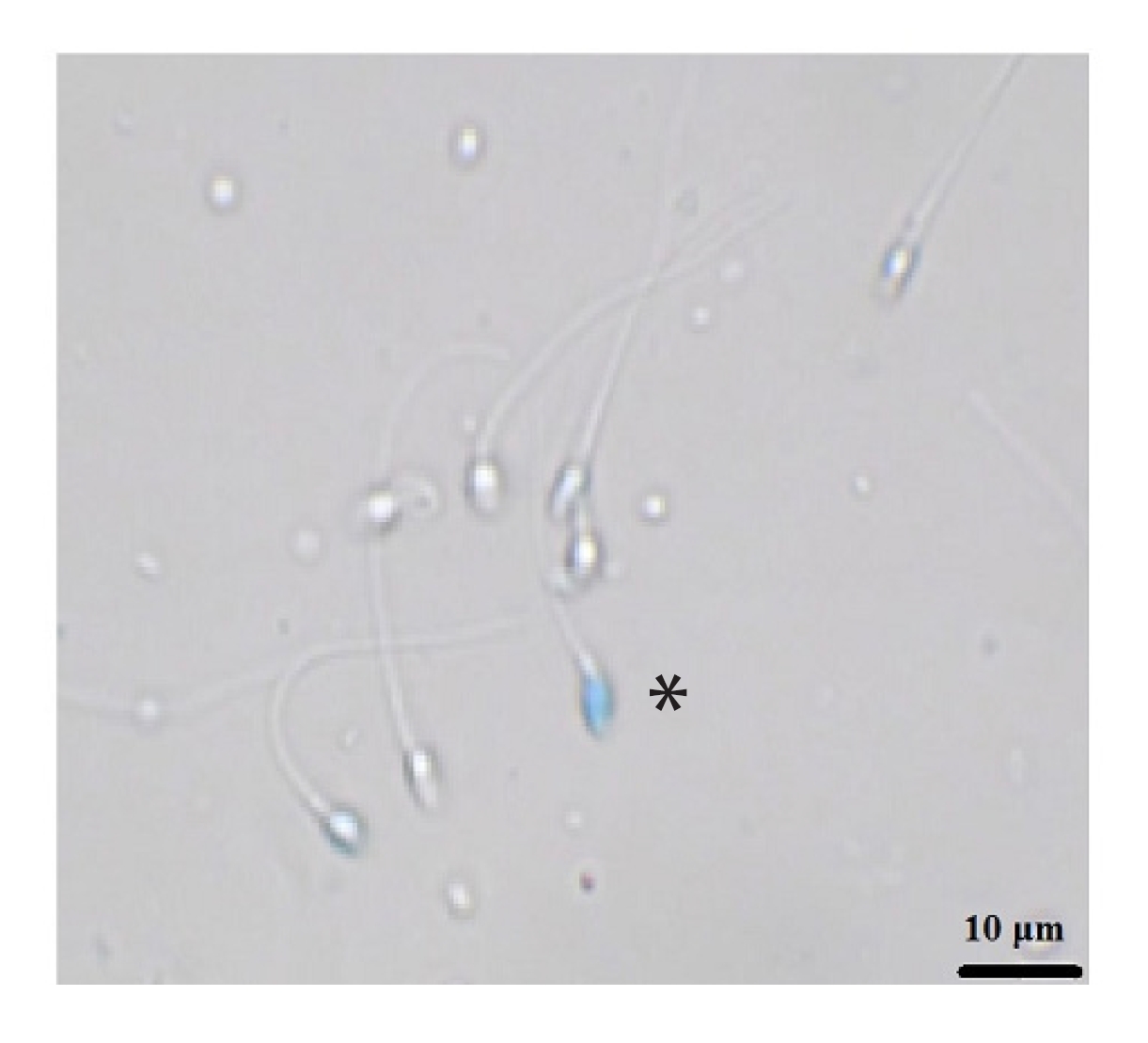
Figure 2 shows the results of the sperm vitality assay by group. There was a significant difference between all freeze-dried groups and the fresh and conventional freezing groups (p<0.0001). However, no significant difference was found among the freeze-dried groups. Figure 3 shows sperm stained with trypan blue. The stained sperm (*) are dead, and unstained sperm are alive.
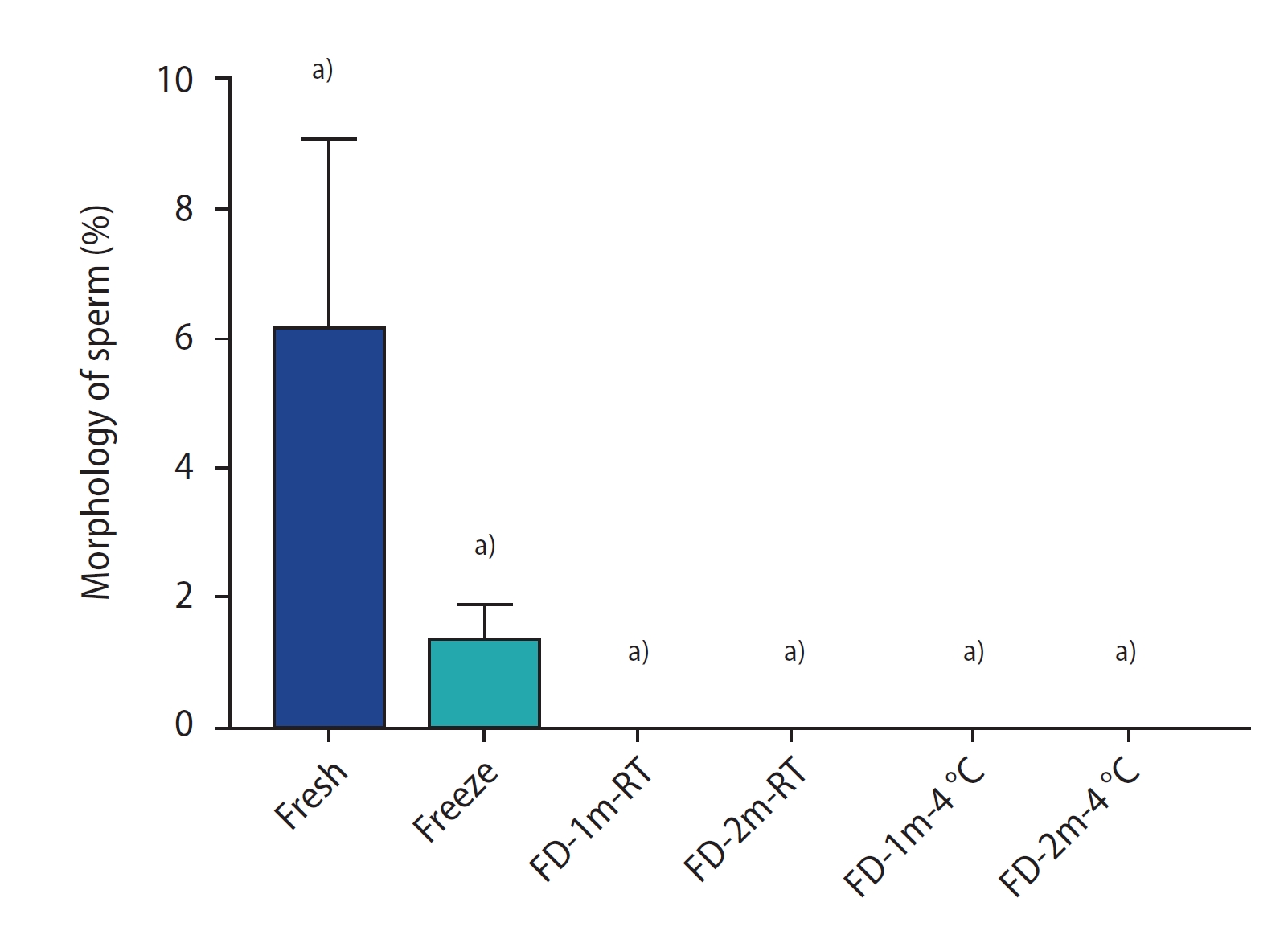
Figure 4 shows the results of the sperm morphology assessment by group. There was a significant difference between all freeze-dried groups and the fresh group (p<0.0001). No significant difference was found between the freeze-dried groups and the freeze group, nor among the freeze-dried groups. In Figure 5, sperm in the FD-1m-RT group were stained with Diff-Quik after being hydrated. Abnormal stained cells were observed with twisted tails or abnormal shapes in the head or neck.
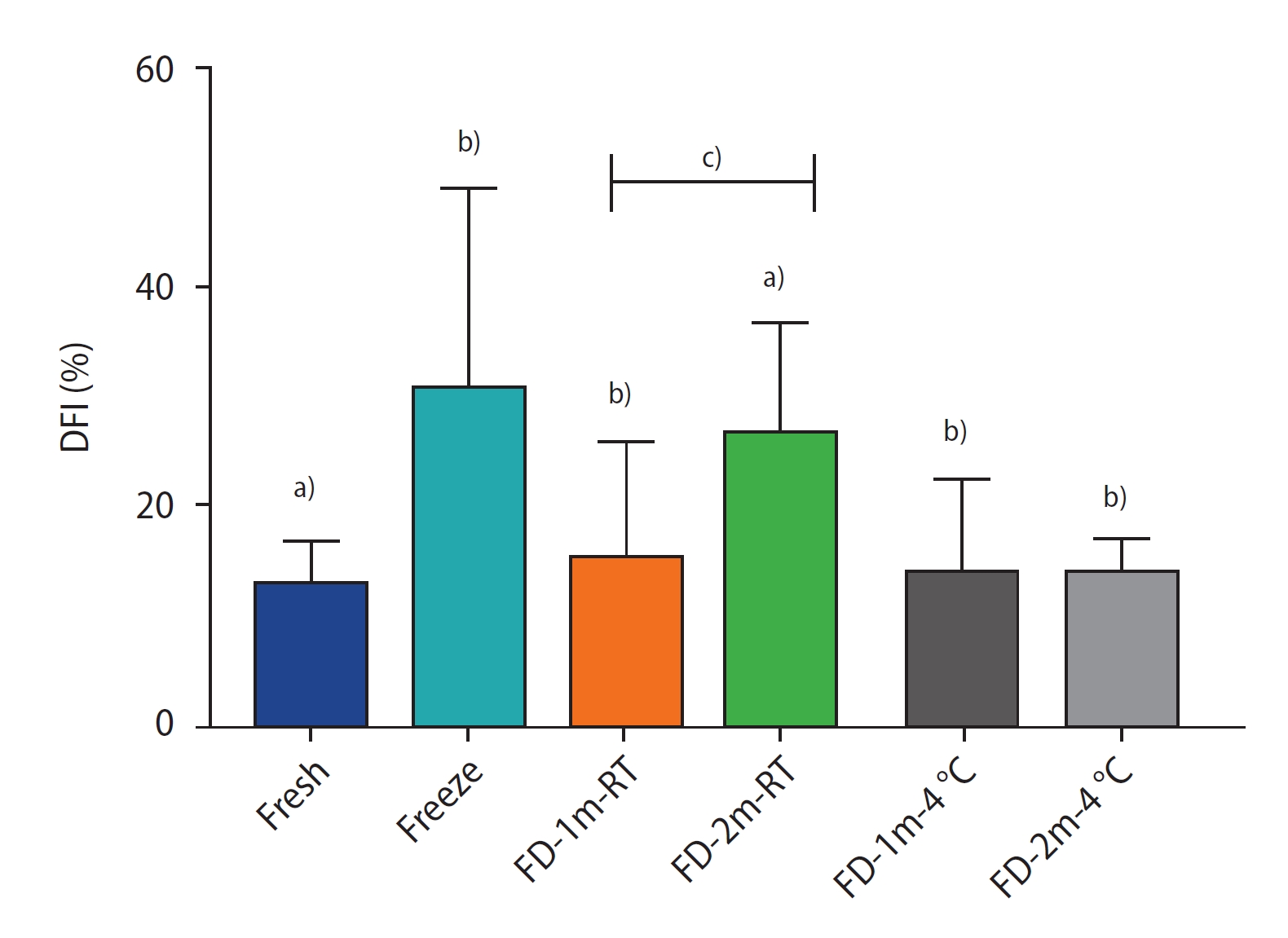
The results of SDFA staining are shown in figure 6. In the comparison of DFI between the freeze- dried groups and the fresh group, except in the FD-2m-RT group (p<0.01), there was no significant difference in the rest of the groups, and freeze-drying did not lead to a significant increase in sperm DFI. In the comparison of DFI between the freeze-dried groups and the freeze group, except in the FD-2m-RT group, there was a significant difference in the rest of the groups (p<0.001), and the DFI in the freeze group showed a significant increase compared to the freeze-dried groups. In the freeze-drying groups at 4┬░C, there was no significant difference between the DFI of the FD-1m-4┬░C and FD-2m-4┬░C groups, but in the freeze-drying group at room temperature, there was a significant difference between the DFI of the FD-1m-RT and FD-2m-RT groups (p<0.05).
Freeze-drying sperm has many potential advantages compared to the freezing method. Storage in LN2 is expensive and requires a constant and guaranteed supply of LN2, regular maintenance, and complex transportation of samples. Freeze-drying overcomes many of these limitations [16].
In our study, in all freeze-dried groups, sperm were immotile after rehydration. Furthermore, the freeze-dried groups had significantly lower sperm vitality than the fresh and freeze groups. Damage caused by freeze-drying to motility and vitality has been reported in previous studies [8,13]. The fact that sperm become immotile after the lyophilization process suggests that they had lost their motility (and vitality) due to stresses associated with the drying or hydration processes. The underlying reasons for damage to cells during lyophilization are still unclear, but since their motility is lost, it is likely that the cell membrane, centriole, and/or other involved mechanisms are damaged [8]. The freeze-dried groups had significantly more morphologically abnormal spermatozoa. This difference, which seemed to be the highest in the tail region, has also been reported in previous studies [17]. A significant increase in tail abnormalities and bent tails has also been reported as a result of hydrating sperm with distilled water [17].
In this study, despite the damage to the sperm cell membrane and motility, the nucleus and its contents remained intact, and freeze-drying did not lead to a significant increase in the sperm DFI, which agrees with previous studies [9,18,19]. Also, in the comparison between the freeze-drying and freezing processes, the DFI was lower in the freeze-dried groups than in the freeze group. It can be concluded that the freeze-drying method causes less damage to sperm DNA than conventional freezing, which may be due to the chelating agents in the freeze-drying liquid, such as ethylene glycoltetraacetic acid (EGTA) and EDTA. The chelating agents prevent the action of sperm endonucleases, which damage the plasma membrane during the freeze-drying process. They prevent sperm from being activated by divalent cations such as Ca2+ and Mg2+, and different chelators seem to be useful in different species. In mice, EDTA is more protective against sperm DNA fragmentation than EGTA [9]. On the contrary, another study showed that EDTA had the same effect as EGTA, but it exerted a greater protective effect against fragmentation and preservation of DNA at different temperatures and time periods [18]. Wakayama and Yanagimachi [7] showed that refrigerating lyophilized mouse sperm and then performing ICSI can produce healthy offspring. Kaneko and Nakagata [20] observed the normal DNA structure of lyophilized mouse sperm at 4 ┬░C [21]. Gianaroli et al. [22] reported that lyophilized human sperm were stored at 4 ┬░C, with no damage to sperm DNA, which is similar to de Lima Bossi's findings [19]. The rate of blastocyst formation in large animals (horses, cows, and pigs) is between 10% and 12%, and in rabbits, it is about 24% [23]. Therefore, although lyophilization significantly damages cell membranes, live birth rates in animal models suggest that this defect has limited functional consequences on fertilization capacity and embryo development [22]. This method also may be more useful for storing sperm with high DFI.
In our study, for the freeze-dried groups at 4 ┬░C, there was no significant difference between the DFIs of the FD-1m-4 ┬░C and FD-2m-4 ┬░C groups, but at room temperature there was a significant difference between the DFIs of the FD-1m-RT and FD-2m-RT groups. This shows that when storing freeze-dried sperm at room temperature, the duration may be relevant and lead to an increase in DFI and damage to sperm DNA. When keeping freeze-dried sperm at 4 ┬░C, however, storage duration may not have an effect, and this storage method is more reliable. Olaciregui et al. [18] observed that the time and temperature conditions of storing different types of freeze-dried dog sperm significantly affected the integrity of their DNA. Additionally, Olaciregui et al. [9], in a mouse study, reported that the maximum DNA fragmentation of freeze-dried sperm samples was directly proportional to the storage temperature. Another study reported that embryos obtained from mouse sperm frozen at 4 ┬░C or 25 ┬░C for up to 3 months had no developmental problems [7]. Therefore, this procedure needs further investigation to optimize the time and temperature conditions.
The limiting factor of sperm lyophilization is the fact that sperm is not motile after the drying process. However, since the DNA remains intact, after rehydration these sperm can be used to fertilize oocytes through ICSI [8]. A limitation of this study was not studying the efficacy of sperm in fertilization with ICSI or evaluating the quality of the resulting embryos due to ethical restrictions.
In this study, both methods of sperm preservation (freeze-drying and freezing in LN2) had negative effects on sperm structure. In particular, the freeze-drying method caused more damage to the cell membrane, yet it better preserved the DNA integrity of the sperm. The temperature and duration of storage were identified as factors that influenced the sperm DFI. Accordingly, more research is necessary to identify methods of improving sperm quality in the freeze-drying process.
Acknowledgments
The authors would like to thank Iran University of Medical Science, Tehran, Iran, for their cooperation throughout the period of study.
Figure┬Ā1.
The percentage of sperm progressive motility of different groups after freezing/thawing or freeze-drying/rehydration. Data are reported as mean┬▒standard deviation. FD-1m-RT, after freeze-drying, preserved at room temperature for 1 month; FD-2m-RT, after freeze-drying, preserved at room temperature for 2 months; FD-1m-4 oC, after freeze-drying, preserved at 4 oC for 1 month; FD-2m-4 oC, after freeze-drying, preserved at 4 oC for 2 months. a) p<0.0001 vs. fresh; b) p<0.0001 vs. freeze.
Figure┬Ā2.
The percentage of vitality staining of sperm of different groups after freezing/thawing or freeze-drying/rehydration. Data are reported as mean┬▒standard deviation. FD-1m-RT, after freeze-drying, preserved at room temperature for 1 month; FD-2m-RT, after freeze-drying, preserved at room temperature for 2 months; FD-1m-4 oC, after freeze-drying, preserved at 4 oC for 1 month; FD-2m-4 oC, after freeze-drying, preserved at 4 oC for 2 months. a)p<0.0001 vs. fresh; b)p<0.0001 vs. freeze.
Figure┬Ā3.
Vitality staining with trypan blue: stained sperm (*) are dead, unstained sperm are alive.
Figure┬Ā4.
The percentage of morphology staining of sperm of different groups after freezing/thawing or freeze-drying/rehydration. Data are reported as mean┬▒standard deviation. FD-1m-RT, after freeze-drying, preserved at room temperature for 1 month; FD-2m-RT, after freeze-drying, preserved at room temperature for 2 months; FD-1m-4 oC, after freeze-drying, preserved at 4 oC for 1 month; FD-2m-4 oC, after freeze-drying, preserved at 4 oC for 2 months. a)p<0.0001 vs. fresh.
Figure┬Ā6.
The percentage of sperm DNA fragmentation index (DFI) of different groups after freezing/thawing or freeze-drying/rehydration. Data are reported as mean┬▒standard deviation. FD-1m-RT, after freeze-drying, preserved at room temperature for 1 month; FD-2m-RT, after freeze-drying, preserved at room temperature for 2 months; FD-1m-4 oC, after freeze-drying, preserved at 4 oC for 1 month; FD-2m-4 oC, after freeze-drying, preserved at 4 oC for 2 months. a)p<0.0001 vs. fresh; b)p<0.0001 vs. freeze; c)p<0.05 vs. FD-1m-RT.
References
1. Amidi F, Pazhohan A, Shabani Nashtaei M, Khodarahmian M, Nekoonam S. The role of antioxidants in sperm freezing: a review. Cell Tissue Bank 2016;17:745-56.



2. Johnson LA, Weitze KF, Fiser P, Maxwell WM. Storage of boar semen. Anim Reprod Sci 2000;62:143-72.


3. Bailey JL, Lessard C, Jacques J, Breque C, Dobrinski I, Zeng W, et al. Cryopreservation of boar semen and its future importance to the industry. Theriogenology 2008;70:1251-9.


4. Lusignan MF, Li X, Herrero B, Delbes G, Chan PT. Effects of different cryopreservation methods on DNA integrity and sperm chromatin quality in men. Andrology 2018;6:829-35.



5. Abolghasemi H, Aghaiipour M, Nikougoftar M, Amirizadeh N, Mohammadi MT, Rahmani S, et al. Leukoreduction in packed cells filtered by home-made. Sci J Iran Blood Transfus Organ 2009;6:13-20.
6. Gil L, Olaciregui M, Luno V, Malo C, Gonzalez N, Martinez F. Current status of freeze-drying technology to preserve domestic animals sperm. Reprod Domest Anim 2014;49 Suppl 4:72-81.


7. Wakayama T, Yanagimachi R. Development of normal mice from oocytes injected with freeze-dried spermatozoa. Nat Biotechnol 1998;16:639-41.



8. Arav A, Saragusty J. Directional freezing of sperm and associated derived technologies. Anim Reprod Sci 2016;169:6-13.


9. Olaciregui M, Luno V, Gonzalez N, Domingo P, de Blas I, Gil L. Chelating agents in combination with rosmarinic acid for boar sperm freeze-drying. Reprod Biol 2017;17:193-8.


10. Polge C, Smith AU, Parkes AS. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature 1949;164:666.



11. Kimura Y, Yanagimachi R. Intracytoplasmic sperm injection in the mouse. Biol Reprod 1995;52:709-20.


12. Asgari F, Gavahi A, Karimi M, Vatannejad A, Amjadi F, Aflatoonian R, et al. Risk of embryo aneuploidy is affected by the increase in sperm DNA damage in recurrent implantation failure patients under ICSI-CGH array cycles. Hum Fertil (Camb) 2022;25:872-80.


13. Abdel Fattah SM, Mohmed HK, Mohamed MA. The potential protective effect of ferulic acid against gamma irradiation induced ovarian failure in rats. Egypt J Radiat Sci Appl 2019;32:1-12.
14. Kashani MH, Ramezani M, Piravar Z. The effect of acrylamide on sperm oxidative stress, total antioxidant levels, tyrosine phosphorylation, and carboxymethyl-lysine expression: a laboratory study. Int J Reprod Biomed 2021;19:625-36.


15. Cheraghi E, Sajadi SM, Soleimani Mehranjani M. The effect of quercetin on the quality of sperm parameters in frozen-thawed semen of patients with Asthenospermia. Andrologia 2021;53:e14167.



16. Kawase Y, Wada NA, Jishage K. Evaluation of DNA fragmentation of freeze-dried mouse sperm using a modified sperm chromatin structure assay. Theriogenology 2009;72:1047-53.


17. Shahmoradi E, Baheiraei N, Halvaei I. Trehalose attenuates detrimental effects of freeze-drying on human sperm parameters. Biopreserv Biobank 2022;20:31-7.


18. Olaciregui M, Luno V, Gonzalez N, De Blas I, Gil L. Freeze-dried dog sperm: dynamics of DNA integrity. Cryobiology 2015;71:286-90.


19. Bossi RL, Cabral M, Oliveira M, Lopes S, Hurtado R, Sampaio M, et al. Ultrastructural analysis of lyophilized human spermatozoa. JBRA Assist Reprod 2021;25:473-9.



20. Kaneko T, Nakagata N. Relation between storage temperature and fertilizing ability of freeze-dried mouse spermatozoa. Comp Med 2005;55:140-4.

21. Olaciregui M, Gil L. Freeze-dried spermatozoa: a future tool? Reprod Domest Anim 2017;52 Suppl 2:248-54.