 |
 |
- Search
| Clin Exp Reprod Med > Volume 50(3); 2023 > Article |
|
Abstract
Objective
Autophagy is highly active in ovariectomized mice experiencing hormone deprivation, especially in the uterine mesenchyme. Autophagy is responsible for the turnover of vasoactive factors in the uterus, which was demonstrated in anti-M├╝llerian hormone receptor type 2 receptor (Amhr2)-Cre-driven autophagy-related gene 7 (Atg7) knockout (Amhr-Cre/Atg7f/f mice). In that study, we uncovered a striking difference in the amount of sequestosome 1 (SQSTM1) accumulation between virgin mice and breeder mice with the same genotype. Herein, we aimed to determine whether repeated breeding changed the composition of mesenchymal cell populations in the uterine stroma.
Methods
All female mice used in this study were of the same genotype. Atg7 was deleted by Amhr2 promoter-driven Cre recombinase in the uterine stroma and myometrium, except for a triangular stromal region on the mesometrial side. Amhr-Cre/Atg7f/f female mice were divided into two groups: virgin mice with no mating history and aged between 11 and 12 months, and breeder mice with at least 6-month breeding cycles with multiple pregnancies and aged around 12 months. The uteri were used for Western blotting and immunofluorescence staining.
Results
SQSTM1 accumulation, representing Atg7 deletion and halted autophagy, was much higher in virgin mice than in breeders. Breeders showed reduced accumulation of several vasoconstrictive factors, which are potential autophagy targets, in the uterus, suggesting that the uterine stroma was repopulated with autophagy-intact cells during repeated pregnancies.
Autophagy is a self-consuming subcellular process that occurs widely in various tissues. While it takes place at a basal level in all tissues, the consequences of autophagy deficiency vary significantly depending on the tissue type. In the mouse uterus, autophagy levels differ between epithelial and mesenchymal tissues [1]. We have previously demonstrated that the uterine mesenchyme, including the stroma, myometrium, and endothelial smooth muscle cells, is a site of active autophagic turnover in mice [2]. Deletion of the upstream autophagy factor, autophagy-related gene 7 (Atg7), which encodes an E1 ubiquitin-activating enzyme, blocks autophagic flux, and is a widely used model system to investigate the role of autophagy [3]. In the mouse uterus, cross-breeding Atg7 floxed mice [3] with anti-M├╝llerian hormone receptor type 2 receptor-Cre (Amhr2-Cre) knock-in mice [4] produces uterine mesenchyme-specific deletion of Atg7, while its expression remains intact in the epithelium. Various vasoactive factors accumulate in Atg7f/f;Amhr2-Cre female mice, leading to hyperpermeability and fluid retention in their uteri [2]. This phenotype is most prominent when steroid hormones are removed via ovariectomy. Random cycling mice also gradually develop similar phenotypes at a slower pace; thus, this phenotype is much more visible in older female mice [2]. The accumulation of vasoactive factors does not interfere with pregnancy, and Atg7f/f;Amhr2-Cre mice can be used at breeders.
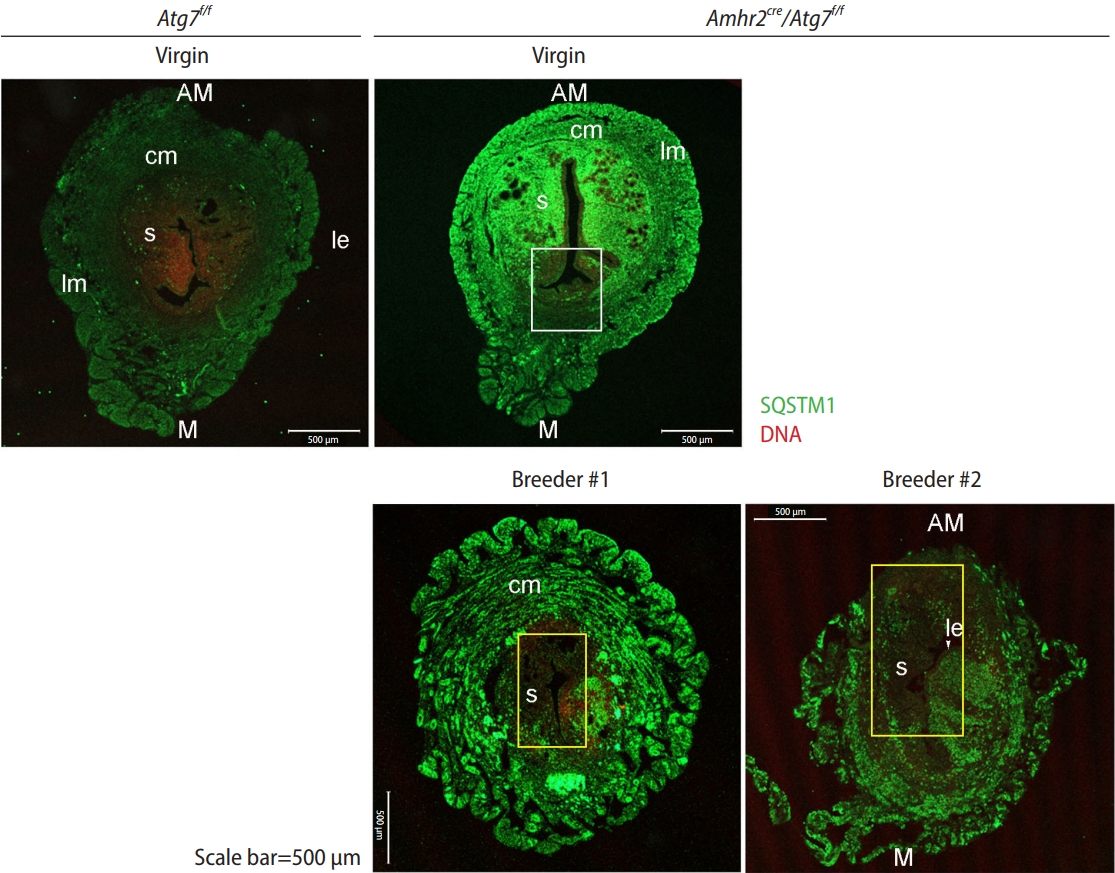
Amhr2-Cre mice have been extensively used to study gene function in the mouse uterus [5-7]. Amh2-Cre removes a floxed gene in the M├╝llerian duct mesenchyme-derived cells in the uterus, but a subset of stromal cells in the mesometrial side (the future site of placental attachment) does not express Amhr2 [8], retaining the floxed gene in this sub-region of the uterine stroma. This area is proportionally quite narrow compared with the Amhr2-active region. In Atg7f/f;Amhr2-Cre uteri, the differing status of gene deletion was visualized using sequestosome 1 (SQSTM1), as an autophagic deficit leads to SQSTM1 buildup in the tissue. As shown in Figure 1, the Atg7-deleted stromal region in the anti-mesometrial side was distinctively marked with anti-SQSTM1 immunofluorescence staining, whereas the myometrial stromal subregion of intact Atg7 showed no SQSTM1 signal (white rectangle) (Figure 1, virgin Atg7f/f;Amhr2-Cre). Thus, this subregion in the mesometrial mesenchyme of the Atg7f/f;Amhr2-Cre uterus retains autophagy and always shows similar patterns of SQSTM1 signal regardless of age [2].
The uterus has a regenerative capacity essential for maintaining homeostasis during repeated reproductive cycles and pregnancy [9]. The mouse endometrium has been shown to retain certain stem cell populations both in the epithelium and stroma [10]. Amhr2-Cre mice crossbred with a reporter gene were previously used to examine how uterine cells regenerate during repeated estrous cycles or postpartum [11]. Stem cells in the epithelial and stromal compartments retain their original fates during repeated estrous cycles, whereas postpartum endometrial regeneration involves a transition from stromal to epithelial cells [11]. This study demonstrated that postpartum uterine regeneration differs from cycle-dependent regeneration during homeotic maintenance of the uterus.
In this study, we examined whether the composition of stromal cells changed after repeated parturition. Following the pattern of SQSTM1 accumulation, we discovered that Atg7-deficient stromal cells were in part replaced by Atg7-intact cells in the uteri of Atg7f/f;Amhr2-Cre breeders, and the phenotype caused by the autophagic deficit seemed to have diminished in them as well.
Amhr2-Cre knock-in mice [4] and Atg7 floxed (Atg7f/f) mice [3] were obtained from Dr. Richard Behringer and the RIKEN BioResource Center (Ibaraki, Japan), respectively. Amhr2-Cre;Atg7f/f mice were crossbred with Institute of Cancer Research mice for more than six generations to obtain mice with better reproductive performance. Amhr2-Cre;Atg7f/f female mice exhibited normal fertility phenotypes equivalent to those of Atg7f/f female mice with respect to the number of deliveries and pups per litter during the breeding period [2]. To compare the status of uterine autophagy between virgins and breeders, Amhr2-Cre;Atg7f/f female mice were separated into virgin (10 to 12 months old) and breeder groups. Virgin mice were not used in breeding and were kept separately from male mice until they reached 10 to 12 months. Breeders, from the ages of 6 to 7 weeks until they reached 10 to 12 months of age, were continuously bred with a stud Atg7f/f male. All mice were maintained according to the policies of the Konkuk University International Animal Care and Use Committee (IACUC approval number KU19078).
Uteri were collected from female mice with the indicated genotypes, cut into 3 to 5 mm pieces, and fixed in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) overnight at 4 ┬░C. The uterine pieces were washed with PBS and embedded in sucrose solution for several hours until the pieces were completely submerged in the solution. They were flash-frozen in optimal cutting temperature compound and subjected to cryosection (12 ╬╝m thickness). The uterine sections were then fixed in 4% PFA-PBS for 20 minutes, washed, and permeabilized with 0.1% Tween-20 in PBS for 20 minutes. The specimens were blocked with 2% bovine serum albumin (BSA) in PBS for 1 hour at 25 ┬░C and then incubated with anti-SQSTM1 antibody (Cell Signaling Technology) in 2% BSA-PBS for 2 hours at 25 ┬░C [2]. After washing, the sections were incubated with Alexa Fluor 488-conjugated secondary antibodies (Thermo Fisher Scientific) for 40 minutes. The sections were counterstained with TOPRO-3-iodide (Thermo Fisher Scientific). The mounted slides were observed by live confocal imaging using a Zeiss LMS900 confocal microscope.
Uteri were collected from each mouse, cut into 5-mm pieces, and placed in lysis buffer containing 10 mM Tris (Thermo Fisher Scientific; pH 7.2), 150 mM NaCl (Thermo Fisher Scientific), 0.1% Triton X-100 (Sigma Aldrich), 5 mM ethylene diamine tetraacetic acid (Thermo Fisher Scientific), 1% sodium dodecyl sulfate (SDS, Thermo Fisher Scientific), 1 mM dithiothreitol (Sigma Aldrich), 1 mM phenylmethylsulfonyl fluoride (MP Biomedicals), and 1├Ś protease inhibitor (Roche). Each sample was homogenized and centrifuged at 15,928 ├Śg for 20 minutes at 4 ┬░C. Protein concentrations were determined by the bicinchoninic acid protein assay (Thermo Fisher Scientific). Lysates (10 ╬╝g) were loaded onto SDS-polyacrylamide gels and transferred onto polyvinylidene difluoride membranes (Sigma Aldrich). The following reagents were used to prepare SDS-polyacrylamide gels: 30% acrylamide mix (Bio-Rad), ammonium persulfate (Sigma Aldrich), TEMED (Sigma Aldrich), 1.5 M Tris (Biosesang; pH 8.8), and 0.5 M Tris (Biosesang; pH 6.8). Chemiluminescent signals were quantified using the ImageQuant LAS4000 system (GE Healthcare) and Multi Gauge software (GE Healthcare). The signal intensity was normalized to the ╬▓-tubulin signal. Data are shown as mean┬▒standard error of the mean of six independent biological samples. The primary antibodies used were anti-SQSTM1, anti-╬▓-catenin (anti-CTNNB1, Cell Signaling Technology), anti-endothelin 1 (anti-EDN1, Biorbyt), anti-nNOS (Cell Signaling Technology), anti-vascular endothelial growth factor A (anti-VEGFA, Abcam), and anti-╬▓-tubulin (Abcam) [2].
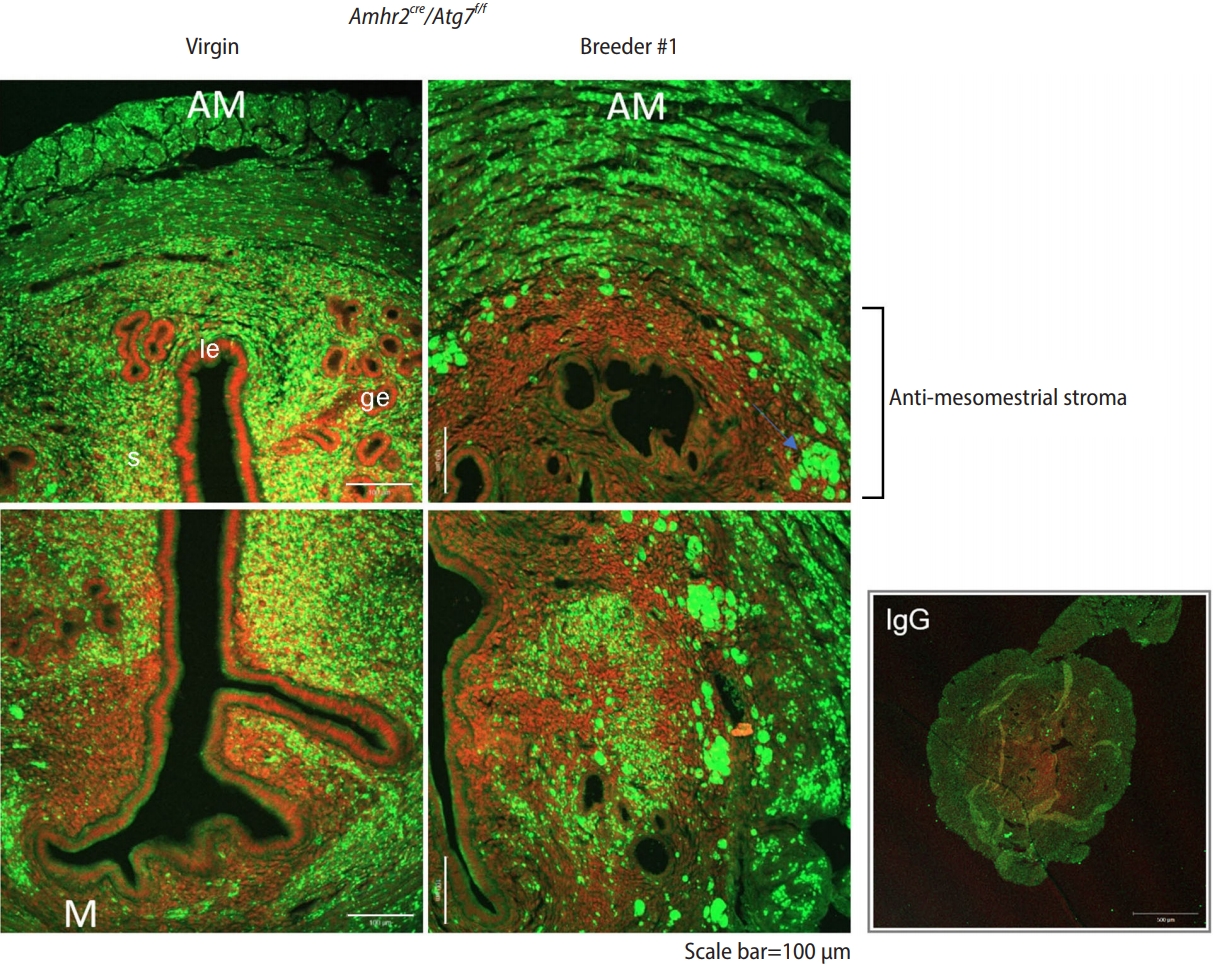
Autophagic defects are generally visualized by examining SQSTM1 accumulation [1] because it is highly upregulated when autophagic flux is blocked [12]. In Atg7f/f (wild-type) virgin uteri, autophagy is generally active, and there was no visible accumulation of SQSTM1 (Figure 1, virgin Atg7f/f), which was consistent with the results of our previous study [2]. In Atg7f/f;Amhr2-Cre random-cycling virgin mice (>11-month-old mice), heavy SQSTM1 buildup was observed in the stroma and myometrium, but not in the luminal and glandular epithelial cells. In addition, the white rectangle marks the small mesenchymal region on the mesometrial side without the SQSTM1 signal, indicating that this area retains Atg7 and intact autophagy. The bottom panel shows uteri from two Atg7f/f;Amhr2-Cre breeders (n=2). In Atg7f/f;Amhr2-Cre breeders, the myometrium was more expanded than in virgin Atg7f/f;Amhr2-Cre mice. Yellow rectangles indicate areas with no SQSTM1 accumulation (Figure 1). In both breeders, the yellow rectangle encompasses the stromal region on the anti-mesometrial and mesometrial sides. The difference in SQSTM1 buildup between Atg7f/f;Amhr2-Cre virgin and breeder mice is clearly visible in Figure 2. The anti-mesometrial side with the supposed Atg7 deletion showed no SQSTM1 accumulation in breeder #1. These results suggest that uterine regeneration after repeated parturition results in the loss of subsets of Atg7-deleted stromal cells.
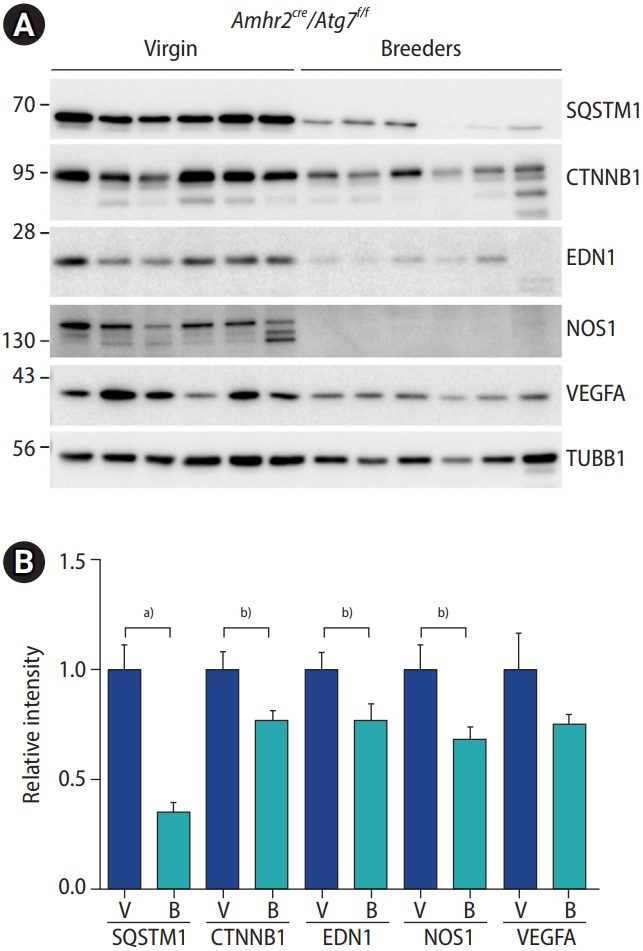
Next, we examined whether the functional aspects of autophagy were restored in the uteri of Atg7f/f and Amhr2-Cre breeders. As previously reported, in Atg7f/f;Amhr2-Cre mice, high levels of several vasoactive factors, including EDN1, nitric oxide synthase 1 (NOS1), VEGFA, and CTNNB1, were observed in their uteri compared to those in wild-type mice. This phenotype was visible in both random cycling and ovariectomized Atg7f/f;Amhr2-Cre uteri [2]. We examined the accumulation of the aforementioned vasoactive factors using Western blotting (Figure 3). Compared to virgin uteri (n=6), the uteri of breeders (n=6) showed significantly reduced accumulation of SQSTM1, CTNNB1, EDN1, NOS1, and VEGFA. These results suggest that postpartum uterine regeneration compensated for autophagy-deficient cells with autophagy-intact cells in the uterine mesenchyme of Atg7f/f;Amhr2-Cre mice.
The uterine mesenchyme is a site of dynamic autophagy, which turns over vasoactive factors during repeated reproductive cycles. When mesenchymal autophagy is blocked by Atg7 deletion, the uterus exhibits hyperpermeability owing to an overload of vasoactive factors [2]. In the present study, we provide evidence that the mesenchymal cell population undergoes a turnover during repeated breeding in Atg7f/f;Amhr2-Cre mice. This observation was possible because of the unique expression profile of Amhr2, which drives Cre expression in this model. Amhr2 is widely expressed in the M├╝llerian duct mesenchyme during development, except for a small subset of mesenchymal cells in the mesometrial side where the broad ligament inserts [11]. When Atg7 is deleted in Amhr2-Cre mice, a subset of mesometrial mesenchymal cells retains the Atg7 gene; thus, the autophagic process is normally preserved in this region [2]. Because SQSTM1 accumulation is only observed in autophagy-deficient cells in the uterus, we were able to follow the potential cell mixing. As shown in Figures 1 and 2, autophagy-deficient mesenchymal cells appeared to have infiltrated the anti-mesometrial side in breeder Atg7f/f;Amhr2-Cre mice (Figures 1 and 2), whereas the uterus of virgin Atg7f/f;Amhr2-Cre mice showed autophagy-deficient mesenchymal cells on the anti-mesometrial side. Cell mixing is possible in the uteri of breeders as the uterus undergoes massive regeneration after parturition in mice [13].
A previous study using the same Amhr2-Cre knock-in mice for the removal of the beta-catenin gene (Ctnnb) in the uterine mesenchyme showed that fat lumps protruded from the anti-mesometrial side of the uterine horns in gene-deleted uteri [14]. It has been suggested that there is a progressive switch in fate from smooth muscle (composing the myometrium) to fat, both of which are of mesenchymal origin [14]. This fate switch was not evident in the mesometrial side where Amhr2-Cre expression was absent. Thus, the Amhr2-Cre knock-in model is useful for delineating the distinct roles of mesenchymal cells in the mesometrial and anti-mesometrial sides of the uterus. In our study, autophagy-deficient mesenchymal cells seem to have been overridden in part by autophagy-intact cells on the mesometrial side (Figures 1 and 2). This change in cell composition further influenced the functional aspect, reducing the burden of autophagic deficit in the whole uterus; that is, a reduction in certain vasoactive factor levels was noted in Atg7f/f;Amhr2-Cre breeders (Figure 3).
The pregnant uterus undergoes massive remodeling postpartum, a process termed uterine involution [15]. In this event of massive tissue remodeling, cell mixing and replenishment are likely to occur. The process involves cell repair by apoptosis, autophagy, extracellular matrix degradation, and proteinase activation, and is believed to involve the activation of certain stem cells [13,15,16]. The label-retaining cell approach has been used to identify the location of endometrial stem cells (ESCs) in the mouse uterus [17]. This approach, following up cells with long-term retention of a DNA synthesis label, identified potential sites of ESCs in the postpartum uterus, such as the subluminal epithelial stroma, near blood vessels, and near the endometrial-myometrial junctions [17]. A similar approach has also identified myometrial stem cells at the periphery of the longitudinal muscle layer [18]. The myometrium in the mouse uterus harbors cells with a quality of stemness [18]. Myocyte proliferation increases in the uteri of pregnant mice, and proliferated myocytes exhibit a synthetic phenotype after parturition, becoming hypertrophic [15]. Myometrial hypertrophy only occurs in gravid uterine horns under hormonal influence [15].
An interesting observation in this study is that mesenchymal cell replenishment occurred from mesometrial autophagy-intact cells to anti-mesometrial autophagy-deficient cells. The remaining question is whether the direction of the cell movement is always the same. This question arises because our mouse model had a clear functional deficit on the anti-mesometrial side. Thus, such deficits may provide guidance for cell mixing and movement. Under normal conditions, when all the mesenchymal cells are functionally equal, cell mixing during tissue repair may follow a distinct pattern. Whether cell mixing has a specific pattern during uterine involution requires further investigation.
Acknowledgments
The authors would like to thank B. Lee and H. Shin for their assistance with this study.
Figure┬Ā1.
Immunofluorescence staining of sequestosome (SQSTM) in uterine sections of virgin mice and breeders. Uterine cryosections (12 ╬╝m) were subjected to immunofluorescence staining using an anti-sequestosome 1 (SQSTM1) antibody. Green signals indicate the sites of SQSTM1 accumulation. All mice were over 11 months of age. Breeders gave birth to multiple litters before use in the experiments, and two independent mice are shown as #1 and #2. Sections were counterstained with TO-PRO-3-iodide (red signal). White rectangle, mesometrial region with no SQSTM1 accumulation and intact autophagy; yellow rectangles, mesenchymal areas with no SQSTM1 accumulation indicating intact autophagy. Scale bar=500 ╬╝m. Atg7, autophagy-related gene 7; Amhr2, anti-M├╝llerian hormone receptor type 2 receptor; AM, anti-mesometrial side; cm, circular muscle; s, stroma; lm, longitudinal muscle; le, luminal epithelim; M, mesometrial side.
Figure┬Ā2.
Enlarged images of uterine sections after sequestosome 1 (SQSTM1) immunofluorescence staining. Representative images of the uteri of virgin and breeder autophagy-related gene 7 (Atg7)f/f; anti-M├╝llerian hormone receptor type 2 receptor (Amhr2)-Cre mice. The virgin uterus showed SQSTM1 accumulation (green) in the muscle and stromal cells, with the exception of the mesometrial side. The breeder uterus (#1) showed an expanded mesenchymal area with no SQSTM1 accumulation, suggesting restored autophagy in this region. AM, anti-mesometrial side; s, stroma; le, luminal epithelim; ge, glandular epithelium; M, mesometrial side; IgG, immunoglobulin G.
Figure┬Ā3.
(A) Western blotting of vasoactive factors in the uteri of anti-M├╝llerian hormone receptor type 2 receptor (Amhr2)-Cre/autophagy-related gene 7 (Atg7)f/f mice. Whole uterine lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting with indicated primary antibodies. (B) The intensity of the chemiluminescence signals from six samples was quantified. ╬▓-Tubulin (TUBB1) values were used to normalize the data. Each group contained six independent samples. Statistical significance was determined using the one-tailed t-test. SQSTM1, sequestosome 1; CTNNB1, ╬▓-catenin; EDN1, endothelin 1; NOS1, nitric oxide synthase; VEGFA, vascular endothelial growth factor A; V, virgin Atg7f/f;Amhr2-Cre mice; B, breeder Atg7f/f;Amhr2-Cre mice. a)p<0.001; b)p<0.05.
References
1. Lim HJ. Autophagy in the uterine vessel microenvironment: balancing vasoactive factors. Clin Exp Reprod Med 2020;47:263-8.




2. Lee B, Shin H, Oh JE, Park J, Park M, Yang SC, et al. An autophagic deficit in the uterine vessel microenvironment provokes hyperpermeability through deregulated VEGFA, NOS1, and CTNNB1. Autophagy 2021;17:1649-66.



3. Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol 2005;169:425-34.




4. Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet 2002;32:408-10.



5. Lague MN, Paquet M, Fan HY, Kaartinen MJ, Chu S, Jamin SP, et al. Synergistic effects of Pten loss and WNT/CTNNB1 signaling pathway activation in ovarian granulosa cell tumor development and progression. Carcinogenesis 2008;29:2062-72.



6. Stewart CA, Wang Y, Bonilla-Claudio M, Martin JF, Gonzalez G, Taketo MM, et al. CTNNB1 in mesenchyme regulates epithelial cell differentiation during M├╝llerian duct and postnatal uterine development. Mol Endocrinol 2013;27:1442-54.



7. Daikoku T, Yoshie M, Xie H, Sun X, Cha J, Ellenson LH, et al. Conditional deletion of Tsc1 in the female reproductive tract impedes normal oviductal and uterine function by enhancing mTORC1 signaling in mice. Mol Hum Reprod 2013;19:463-72.



8. Arango NA, Kobayashi A, Wang Y, Jamin SP, Lee HH, Orvis GD, et al. A mesenchymal perspective of M├╝llerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev 2008;75:1154-62.


9. Gargett CE, Chan RW, Schwab KE. Hormone and growth factor signaling in endometrial renewal: role of stem/progenitor cells. Mol Cell Endocrinol 2008;288:22-9.


10. Chan RW, Gargett CE. Identification of label-retaining cells in mouse endometrium. Stem Cells 2006;24:1529-38.



11. Huang CC, Orvis GD, Wang Y, Behringer RR. Stromal-to-epithelial transition during postpartum endometrial regeneration. PLoS One 2012;7:e44285.



12. Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016;12:1-222.


13. Spooner MK, Lenis YY, Watson R, Jaimes D, Patterson AL. The role of stem cells in uterine involution. Reproduction 2021;161:R61-77.


14. Arango NA, Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol 2005;288:276-83.


15. Shynlova O, Tsui P, Jaffer S, Lye SJ. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur J Obstet Gynecol Reprod Biol 2009;144 Suppl 1:S2-10.


16. Hsu KF, Pan HA, Hsu YY, Wu CM, Chung WJ, Huang SC. Enhanced myometrial autophagy in postpartum uterine involution. Taiwan J Obstet Gynecol 2014;53:293-302.


- TOOLS







