 |
 |
- Search
| Clin Exp Reprod Med > Volume 50(3); 2023 > Article |
|
Abstract
Monospermy occurs in the process of normal fertilization where a single sperm fuses with the egg, resulting in the formation of a diploid zygote. During the process of fertilization, the sperm must penetrate the zona pellucida (ZP), the outer layer of the egg, to reach the egg’s plasma membrane. Once a sperm binds to the ZP, it undergoes an acrosomal reaction, which involves the release of enzymes from the sperm's acrosome that help it to penetrate the ZP. Ovastacin is one of the enzymes that is involved in breaking down the ZP. Studies have shown that ovastacin is necessary for the breakdown of the ZP and for successful fertilization to occur. However, the activity of ovastacin is tightly regulated to ensure that only one sperm can fertilize the egg. One way in which ovastacin helps to prevent polyspermy (the fertilization of an egg by more than one sperm) is by rapidly degrading the ZP after a sperm has penetrated it. This makes it difficult for additional sperm to penetrate the ZP and fertilize the egg. Ovastacin is also thought to play a role in the block to polyspermy, a mechanism that prevents additional sperm from fusing with the egg's plasma membrane after fertilization has occurred. In summary, the role of ovastacin in monospermic fertilization is to help ensure that only one sperm can fertilize the egg, while preventing polyspermy and ensuring successful fertilization.
Hormonal contraceptives work by altering the hormonal balance in the body to prevent ovulation and inhibit fertilization [1]. However, they can also have a range of side effects. These include an increased risk of blood clots, stroke, and heart attack, as well as headaches, mood changes, and weight gain [2]. Other non-hormonal approaches being investigated include the use of natural compounds that can inhibit sperm function or fertilization, such as plant-based compounds and peptides [3,4]. Research is also being conducted on the development of vaccines that could stimulate the immune system to produce antibodies against sperm or other components of the reproductive process [5].
During fertilization in humans, the sperm penetrates the oocyte and fuses with the egg cell. This process triggers a series of biochemical events, including the release of cortical granules from the egg [6]. Cortical granules are organelles that are present in the cortex, or outer layer, of the egg. When fertilization occurs, the cortical granules release their contents into the perivitelline space, which is the area between the egg cell membrane and the zona pellucida (ZP), a layer of glycoproteins that surrounds the egg. One of the proteins released by the cortical granules is ovastacin, which belongs to the astacin family of metalloproteinases [7]. Ovastacin is involved in the process of fertilization by cleaving the N-terminal of the ZP2 protein in the ZP. This cleavage prevents other sperm from binding to the ZP and fertilizing the egg, thus preventing polyspermy. In addition to the release of cortical granules and ovastacin, studies have also shown that a "zinc spark" occurs during fertilization in mice and humans [8]. The release of zinc ions is thought to be involved in the process of hardening the ZP by modifying the microarchitecture of the ZP matrix. Overall, the process of fertilization involves a complex series of biochemical events, including the release of cortical granules and the cleavage of ZP2 by ovastacin, as well as the release of zinc ions that contribute to the hardening of the ZP [9].
In this context, we reviewed the impact of ovastacin on the prevention of polyspermy by changing the structure of ZP on oocytes and blocking the entrance of sperm. We also produced a recombinant protein of mouse ovastacin to further study its biological function. Ovastacin could be a beneficial and effective contraceptive in terms of its physiologically non-hormonal mechanism of action that is risk-free compared to ingesting hormonal pills if it is administered correctly.
Fertilization in mammals requires the fusion of the nuclei of the sperm and oocyte, and the binding of the sperm to the ZP surrounding the oocyte. To prevent polyspermy, the ZP undergoes biochemical modification to prevent other sperm from penetrating and fusing with the oocyte [9]. One of the key components of this modification is ovastacin, which is encoded by the astacin-like metalloendopeptidase (ASTL) gene and is a metalloproteinase belonging to the astacin family [10]. Ovastacin is specifically expressed in the oocyte at or beyond the secondary follicle stage, and is located in the plasma membrane and cortical granules of the oocyte [11]. After fertilization, ovastacin is released from the cortical granules and cleaves the N-terminal of the ZP2 protein in the ZP, preventing other sperm from binding to and fertilizing the oocyte. The catalytic site of ovastacin reacts with zinc ions to activate the enzyme. Ovastacin is composed of several domains, including a signal peptide, a pro-peptide, a proteinase domain with a catalytic site, and a C-terminal region (CTR). The CTR contains a specific sequence for each species and is located outside the plasma membrane [10,11]. Ovastacin plays a critical role in preventing polyspermy during fertilization in mammals, and its location and structure have been studied in detail to better understand its function in this process.
There are two major known functions of ovastacin. One is related to fertilization, and the other is related to the development of the follicle [12]. Normally, one egg and one sperm must meet to form one zygote, so that normal embryonic development proceeds. When more than one sperm enters an egg, it is called polyspermy. In order to prevent polyspermy, structural changes occur in the ovum transparent ZP during the fertilization process. First, one sperm binds to the N-terminal of ZP2, one of the structures of ZP, the egg is activated and zinc ions in the cortical granule are immediately released [13]. It was thought that there would be an interaction between zinc ions and ovastacin at the same location in the egg, and it was confirmed that the concentration of ovastacin decreases after the zinc spark actually occurs [8]. In addition, when zinc ions are exposed to the ZP, ovastacin is able to modify the microstructure by increasing the density and fiber thickness of the ZP [14]. Following zinc reaction with ovastacin to modify the ZP, the attachment of sperm to the egg dramatically declines [15]. Therefore, zinc is thought to be an ion that plays an important role in hardening the ZP through the secretion of cortical granules. After zinc ion exocytosis, ovastacin released from cortical granules binds to the N-terminal of ZP2 and then finally cleaves ZP2. By confirming the attachment of sperm in two-cell embryos with untruncated ZP2, it can be seen that the structural change of ZP2 is important in polyspermy [16]. That is, polyspermy can be prevented through ZP hardening, which is caused by the release and activation of ovastacin located in the cortical granule of the egg [17]. The second function of ovastacin is related to the development of the follicle [12]. Ovastacin is specifically expressed in the oocyte at or beyond the secondary follicle stage [11,18]. During follicular development, the ZP matrix of the oocyte undergoes continuous remodeling to accommodate the growing oocyte. This remodeling is mediated by several enzymes, including ovastacin, which cleaves ZP2 during the final stages of follicular development. Cleavage of ZP2 by ovastacin is thought to be important for the removal of cumulus cells that surround the oocyte and for the release of the oocyte from the follicle during ovulation [19]. In addition, ovastacin has been shown to be involved in the expansion of cumulus cells, which are important for the development and maturation of the oocyte [20,21]. Collectively, ovastacin plays a crucial role in both fertilization and follicular development in mammals.
The ZP is a glycoprotein structure that surrounds the mammalian oocyte and is composed of three or four different proteins depending on the species. The glycoproteins of the ZP are synthesized, secreted, and assembled by growing oocytes. The fibrils, which are in the inner or outer regions of the ZP, are arranged perpendicular and parallel to the oolemma, giving the ZP a multilayered appearance. The ZP is composed of two subdomains, ZP-N and ZP-C, separated by a short linker region. Both subdomains adopt immunoglobulin-like folds for their three-dimensional structure and play an essential role in the polymerization of nascent ZP proteins with crosslinked fibrils. In humans, ZP proteins are composed of four glycoproteins (ZP1, ZP2, ZP3, and ZP4) and surround a 120-μm oocyte, while in mice, the ZP is composed of ZP1, ZP2, and ZP3 and surrounds an ovulated oocyte that is 80 μm in diameter. The ZP plays a crucial role in fertilization, and multiple ZP proteins bind to spermatozoa in humans. Among ZP1 to ZP4, ZP2 binds to the acrosome associated with primary spermatozoa in both humans and mice. When the proteolytic cleavage of ZP2 occurs in the acrosome reaction, its microstructure is modified to prevent the binding of multiple sperm. In humans, ZP1, ZP3, and ZP4 bind to the capacitated human spermatozoa and induce an acrosome reaction, while in mice, ZP3 acts as the primary sperm receptor. O-linked glycans of ZP3 are more relevant to the induction of the acrosome reaction in mice, while N-linked glycans of ZP3 and ZP4 are critical in humans. Recent studies suggest that O-linked glycans and N-linked glycans of human ZP play a critical role in the sperm-egg binding system. Therefore, mutations in the ZP genes can lead to abnormal ZP glycoproteins, resulting in an abnormal ZP matrix and eventually leading to subfertility in women.
Mutations in the ASTL gene have been studied in various mammalian species, including humans and mice [22]. In mice, genetic studies have shown that the absence of ovastacin can lead to impaired fertilization, reduced egg viability, and subfertility [10,13,14].
Mutations in ZP genes can lead to various reproductive disorders [23,24]. The ZP genes encode the glycoproteins that form the extracellular matrix surrounding the oocyte, which plays a critical role in fertilization. Mutations in ZP genes can result in abnormal ZP structure, leading to infertility or subfertility in females. For example, mutations in the ZP1 gene have been associated with rare cases of female infertility [25,26]. In these cases, affected individuals have an abnormal ZP structure, resulting in failed fertilization or early embryonic loss. Mutations in the ZP2 gene have also been linked to infertility in some cases [27]. In addition to infertility, mutations in the ZP genes have been associated with other reproductive disorders, such as ovarian dysgenesis and premature ovarian failure. These conditions are characterized by abnormal ovarian development and function, resulting in reduced fertility or early menopause. Mutations in the ZP genes can have a significant impact on female reproductive health and fertility [26,27]. Genetic testing and counseling can be helpful for individuals with a family history of reproductive disorders or those experiencing infertility or subfertility.
The mouse ovastacin gene (GenBank: AJ537599.2) was synthesized by Bioneer. The gene (64S–432D), except for the signal peptide, was amplified by polymerase chain reaction using primers (forward: 5' ATCGAGGATCCTTCAGAAGAGACCCCAGAATCC 3'; reverse: 5' ATTACGCGGCCGCTATCACGGGGCACTTCTCTAAT 3') and cloned into modified pAcGP67a, as shown in Figure 1.
Fetuin is a liver-derived plasma protein belonging to the superfamily of cystatin proteins. Fetuin is divided into A and B, but the structure is similar, with two cystatin-like domains (CLDs) and a third domain whose function is unknown . Both fetuin-A and fetuin-B mRNAs are highly expressed in the liver and, to a lesser extent, in the tongue and placenta [28]. In addition, small amounts are expressed in various organs [28]. The concentration of fetuin-B was not found to differ significantly between ovarian follicular fluid and plasma [29]. Interestingly, fetuin-B, a liver-derived plasma protein, is thought to be an essential factor for successful fertilization [30,31].
Fetuin-A is a member of the cystatin superfamily of proteins, but it has a different structure compared to fetuin-B. It contains two CLDs connected by a flexible linker region [32]. The N-terminal CLD of fetuin-A has been shown to inhibit calcium phosphate precipitation and growth, and this activity is thought to play a role in preventing pathological calcification in tissues. Fetuin-A has also been found to interact with several ligands, including transforming growth factor-beta, bone morphogenetic proteins, and insulin [33,34]. It has been proposed that fetuin-A acts as a carrier protein for these ligands, protecting them from proteolytic degradation and facilitating their transport to target cells. Mutations in the alpha 2-Heremans Schmid glycoprotein (AHSG) gene that encodes fetuin-A have been associated with various diseases, such as insulin resistance, obesity, and metabolic syndrome [32]. In addition, decreased levels of fetuin-A have been observed in several diseases, including liver disease, chronic kidney disease, and cardiovascular disease [35].
Fetuin-B consists of a cysteine-like domain and a CTR. Cysteine 1 (CY1) and cysteine 2 (CY2) are cysteine-like domains that are connected to the CTR in the CPDCP (C151-P155) trunk structure, which act as a pathway to the active site of metallopeptidase of the astacin family [31,36]. Cysteine-like proteins are known as inhibitors of cysteine proteases. Fetuin-B inhibits metallopeptidases of the astacin family [31]. Unlike other cysteine-like proteins, fetuin-A does not inhibit cysteine proteases. Fetuin-B reacts specifically to metallopeptidases in the astacin superfamily and inhibits cysteine protease. Specifically, fetuin-B inhibits the function of ovastacin [31]. When a sufficient amount of fetuin-B is present in serum, ovastacin binds to fetuin-B prior to sperm and egg binding, which may prevent premature ZP hardening [30,37]. However, if the concentration of fetuin-B in serum is low, the free forms of ovastacin are responsible for cleaving ZP2, and then premature ZP hardening may occur, preventing fertilization even for a competent sperm and egg. Briefly, ovastacin protects the embryo and prevents polyspermy when it is produced in an oocyte bound with sperm, but when it acts on an unbound oocyte, it causes ZP cleavage of the oocyte before fertilization and prevents fertilization. When recombinant fetuin-B protein was injected into the body of a mouse model with a lower than average serum concentration of fetuin-B, a previous study demonstrated that the fertility rate increased by preventing premature ZP hardening [19,30]. Preventing premature ZP hardening through methods such as adding fetuin-B to the culture medium in vitro fertilization (IVF) seems to be helpful in improving the fertility rate [29]. Therefore, fetuin-B is expected to be helpful in improving the fertility rate at IVF clinics.
It is important to note that the development of non-hormonal contraceptives is crucial for providing women with alternative options for contraception. While inhibiting fertilization through the inactivation of sperm has been a focus of many efforts to develop contraceptive molecules, the use of recombinant ovastacin to prevent fertilization by prematurely hardening the ZP has shown some potential. However, further studies are required to determine the effectiveness of this method and to explore ways to enhance its contraceptive ability. It is also important to continue exploring other physiologically-based approaches to contraception to provide women with a range of safe and effective options.
Although the current study did not find a significant biological function of recombinant mouse ovastacin, this does not rule out the potential for ovastacin as a contraceptive molecule. More research is needed to fully understand the mechanism of action and effectiveness of ovastacin as a contraceptive. Additionally, further studies may explore the potential use of ovastacin in other reproductive contexts, such as assisted reproductive technologies or fertility treatments. This study provides valuable insights into the role of ovastacin in fertilization and highlights the need for continued research in this area.
Notes
Author contributions
Conceptualization: IK, MK, HY, BSP, JHJ, JL. Dara curation: IK, MK, HY, BSP, JHJ, JL. Formal analysis: IK, MK, HY, BSP, JHJ, JL. Funding acquisition: JHJ, JL. Methodology: IK, MK, HY, BSP, JHJ, JL. Project administration: BSP, JHJ, JL. Visualization: IK, MK, HY, BSP, JHJ, JL. Writing-original draft: IK, MK. Writing-review & editing: JL.
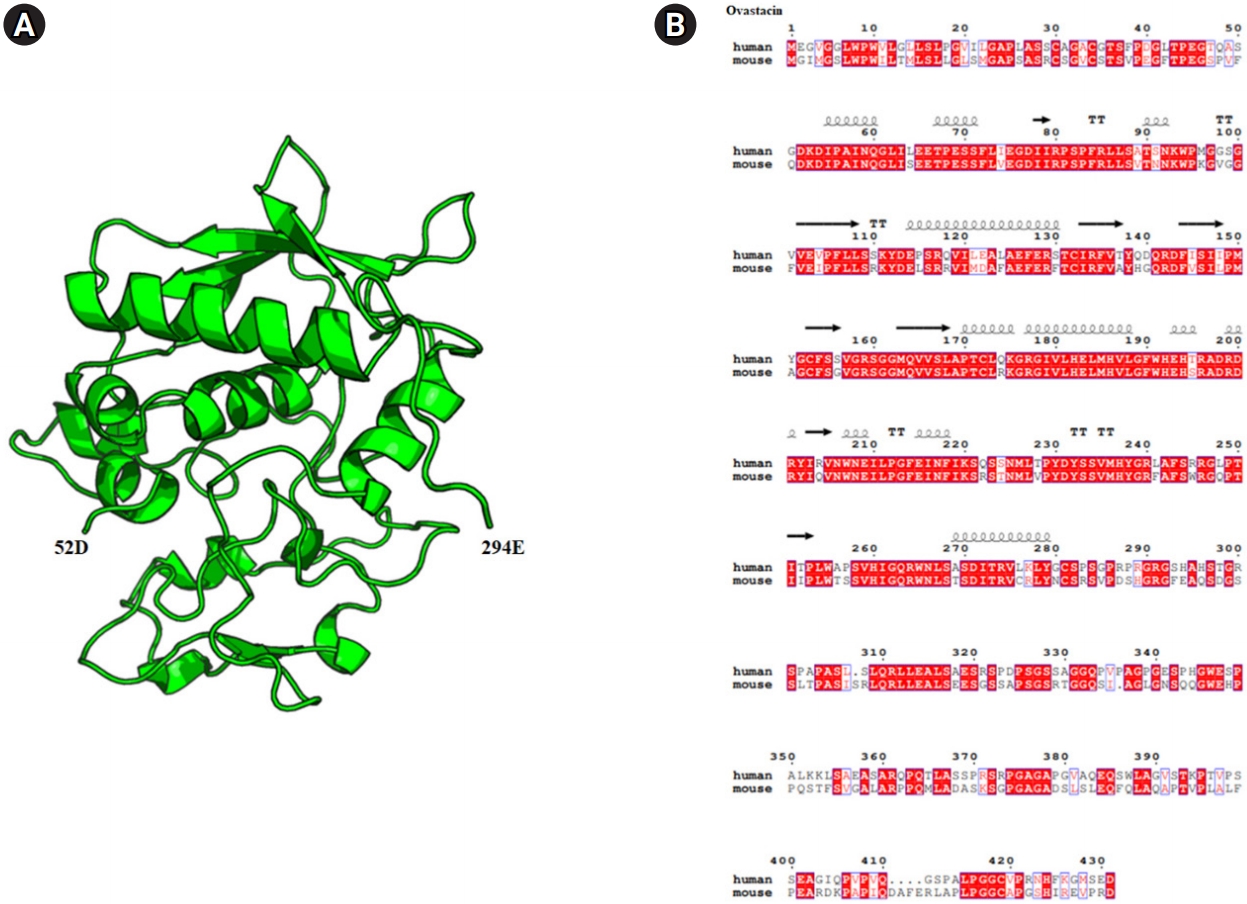
Figure 1.
Diagram of the crystal structure and sequence alignment of human and mouse ovastacin. (A) Mouse ovastacin model (52D–294E) generated using the Swiss model program (https://swissmodel.expasy.org/). The overall structure is represented in a cartoon style with α-helices and the β-strands, which are numbered from the N to C terminus using the PyMOL program (https://pymol.org). (B) Ovastacin model structure-based sequence alignment of mouse ovastacin with human ovastacin (https://www.ebi.ac.uk/Tools/msa/clustalo/, https://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi).
References
1. Harrison D, Buskmiller C, Chireau M, Ruppersberger LA, Yeung PP Jr. Systematic review of ovarian activity and potential for embryo formation and loss during the use of hormonal contraception. Linacre Q 2018;85:453-69.




2. Teal S, Edelman A. Contraception selection, effectiveness, and adverse effects: a review. JAMA 2021;326:2507-18.


3. Casado-Espada NM, de Alarcon R, de la Iglesia-Larrad JI, Bote-Bonaechea B, Montejo AL. Hormonal contraceptives, female sexual dysfunction, and managing strategies: a review. J Clin Med 2019;8:908.



4. Kent K, Johnston M, Strump N, Garcia TX. Toward development of the male pill: a decade of potential non-hormonal contraceptive targets. Front Cell Dev Biol 2020;8:61.



5. Naz RK. Vaccine for human contraception targeting sperm Izumo protein and YLP12 dodecamer peptide. Protein Sci 2014;23:857-68.




6. Liu M. The biology and dynamics of mammalian cortical granules. Reprod Biol Endocrinol 2011;9:149.



7. Korschgen H, Jager C, Tan K, Buchholz M, Stocker W, Ramsbeck D. A primary evaluation of potential small-molecule inhibitors of the astacin metalloproteinase ovastacin, a novel drug target in female infertility treatment. ChemMedChem 2020;15:1499-504.




8. Duncan FE, Que EL, Zhang N, Feinberg EC, O’Halloran TV, Woodruff TK. The zinc spark is an inorganic signature of human egg activation. Sci Rep 2016;6:24737.




9. Tokuhiro K, Dean J. Glycan-independent gamete recognition triggers egg zinc sparks and ZP2 cleavage to prevent polyspermy. Dev Cell 2018;46:627-40.



10. Korschgen H, Kuske M, Karmilin K, Yiallouros I, Balbach M, Floehr J, et al. Intracellular activation of ovastacin mediates pre-fertilization hardening of the zona pellucida. Mol Hum Reprod 2017;23:607-16.



11. Pires ES, Hlavin C, Macnamara E, Ishola-Gbenla K, Doerwaldt C, Chamberlain C, et al. SAS1B protein [ovastacin] shows temporal and spatial restriction to oocytes in several eutherian orders and initiates translation at the primary to secondary follicle transition. Dev Dyn 2013;242:1405-26.



12. Sachdev M, Mandal A, Mulders S, Digilio LC, Panneerdoss S, Suryavathi V, et al. Oocyte specific oolemmal SAS1B involved in sperm binding through intra-acrosomal SLLP1 during fertilization. Dev Biol 2012;363:40-51.



13. Xiong B, Zhao Y, Beall S, Sadusky AB, Dean J. A unique egg cortical granule localization motif is required for ovastacin sequestration to prevent premature ZP2 cleavage and ensure female fertility in mice. PLoS Genet 2017;13:e1006580.



14. Burkart AD, Xiong B, Baibakov B, Jimenez-Movilla M, Dean J. Ovastacin, a cortical granule protease, cleaves ZP2 in the zona pellucida to prevent polyspermy. J Cell Biol 2012;197:37-44.




15. Que EL, Duncan FE, Bayer AR, Philips SJ, Roth EW, Bleher R, et al. Zinc sparks induce physiochemical changes in the egg zona pellucida that prevent polyspermy. Integr Biol (Camb) 2017;9:135-44.



16. Avella MA, Xiong B, Dean J. The molecular basis of gamete recognition in mice and humans. Mol Hum Reprod 2013;19:279-89.



17. Vogt EJ, Tokuhiro K, Guo M, Dale R, Yang G, Shin SW, et al. Anchoring cortical granules in the cortex ensures trafficking to the plasma membrane for post-fertilization exocytosis. Nat Commun 2019;10:2271.




18. de Figueiredo JR, de Lima LF, Silva JR, Santos RR. Control of growth and development of preantral follicle: insights from in vitro culture. Anim Reprod 2018;15(Suppl 1): 648-59.



19. Dietzel E, Floehr J, Van de Leur E, Weiskirchen R, Jahnen-Dechent W. Recombinant fetuin-B protein maintains high fertilization rate in cumulus cell-free mouse oocytes. Mol Hum Reprod 2017;23:25-33.


20. Ducibella T, Kurasawa S, Rangarajan S, Kopf GS, Schultz RM. Precocious loss of cortical granules during mouse oocyte meiotic maturation and correlation with an egg-induced modification of the zona pellucida. Dev Biol 1990;137:46-55.


21. Schiewe MC, Araujo E Jr, Asch RH, Balmaceda JP. Enzymatic characterization of zona pellucida hardening in human eggs and embryos. J Assist Reprod Genet 1995;12:2-7.



22. Maddirevula S, Coskun S, Al-Qahtani M, Aboyousef O, Alhassan S, Aldeery M, et al. ASTL is mutated in female infertility. Hum Genet 2022;141:49-54.



23. Wassarman PM, Litscher ES. Zona pellucida genes and proteins: essential players in mammalian oogenesis and fertility. Genes (Basel) 2021;12:1266.



25. Wang J, Yang X, Sun X, Ma L, Yin Y, He G, et al. A novel homozygous nonsense mutation in zona pellucida 1 (ZP1) causes human female empty follicle syndrome. J Assist Reprod Genet 2021;38:1459-68.




26. Cao Q, Zhao C, Zhang X, Zhang H, Lu Q, Wang C, et al. Heterozygous mutations in ZP1 and ZP3 cause formation disorder of ZP and female infertility in human. J Cell Mol Med 2020;24:8557-66.




27. Zhou Z, Ni C, Wu L, Chen B, Xu Y, Zhang Z, et al. Novel mutations in ZP1, ZP2, and ZP3 cause female infertility due to abnormal zona pellucida formation. Hum Genet 2019;138:327-37.



28. Denecke B, Graber S, Schafer C, Heiss A, Woltje M, Jahnen-Dechent W. Tissue distribution and activity testing suggest a similar but not identical function of fetuin-B and fetuin-A. Biochem J 2003;376(Pt 1): 135-45.




29. Fang L, Hu X, Cui L, Lv P, Ma X, Ye Y. Serum and follicular fluid fetuin-B levels are correlated with fertilization rates in conventional IVF cycles. J Assist Reprod Genet 2019;36:1101-7.




30. Dietzel E, Wessling J, Floehr J, Schafer C, Ensslen S, Denecke B, et al. Fetuin-B, a liver-derived plasma protein is essential for fertilization. Dev Cell 2013;25:106-12.


31. Karmilin K, Schmitz C, Kuske M, Korschgen H, Olf M, Meyer K, et al. Mammalian plasma fetuin-B is a selective inhibitor of ovastacin and meprin metalloproteinases. Sci Rep 2019;9:546.




32. Chekol Abebe E, Tilahun Muche Z, Behaile T/Mariam A, Mengie Ayele T, Mekonnen Agidew M, Teshome Azezew M, et al. The structure, biosynthesis, and biological roles of fetuin-A: a review. Front Cell Dev Biol 2022;10:945287.



33. Bourebaba L, Marycz K. Pathophysiological implication of fetuin-A glycoprotein in the development of metabolic disorders: a concise review. J Clin Med 2019;8:2033.



34. Shim YS, Kang MJ, Oh YJ, Baek JW, Yang S, Hwang IT. Fetuin-A as an alternative marker for insulin resistance and cardiovascular risk in prepubertal children. J Atheroscler Thromb 2017;24:1031-8.



35. Pan X, Wen SW, Bestman PL, Kaminga AC, Acheampong K, Liu A. Fetuin-A in metabolic syndrome: a systematic review and meta-analysis. PLoS One 2020;15:e0229776.



- TOOLS







