 |
 |
- Search
| Clin Exp Reprod Med > Volume 49(4); 2022 > Article |
|
Abstract
Objective
The present study assessed the biological characteristics of human spermatozoa at different time intervals (0, 1, 1.5, and 2 hours) after incubation at 37°C.
Methods
Twenty-five normozoospermic semen samples were incubated at 37°C. Incubation was performed at four time intervals of 0 (after liquefaction), 1, 1.5, and 2 hours. The samples were evaluated for sperm parameters at each time interval.
Results
The rate of sperm progressive motility decreased at 1.5 hours compared to 0 hours as well as 2 hours compared to 1 hour and 0 hours. The rate of non-motile spermatozoa also decreased after 2 hours compared to after 0 hours. No significant changes were observed in sperm viability (p=0.98) and non-progressive motility (p=0.48) at any time intervals. Abnormal sperm morphology increased at 1.5 hours of incubation time (p<0.001). No significant changes were observed in DNA fragmentation at 1 hour compared to 0 hours (median [interquartile range]: 19.5 [4] vs. 19 [4]), as well as at 1.5 hours compared to 1 hour (20 [5]). However, a significant increase in DNA fragmentation was observed at 1.5 hours compared to 0 hours. The mitochondrial membrane potential decreased remarkably after 1 hour of incubation time. No significant differences were observed in the acrosome reaction or malonaldehyde levels at any time point (p=0.34 and p=0.98, respectively).
Infertility or low fertility refers to the inability of couples to achieve pregnancy for 1 year, despite regular unprotected sexual intercourse, according to the World Health Organization [1]. About 15% of couples have infertility-related problems and seek medical treatment for infertility [2]. In general, 20% to 50% of infertility cases are related to spermatozoa parameters [3]. At many clinics, common semen parameters, such as count, morphology, and motility, are used to assess sperm quality. The seminal fluid that is delivered to the laboratory for analysis and use in assisted reproductive technology (ART) must be sampled and stored under special conditions based on the World Health Organization (WHO) protocol [4].
One of the most important factors affecting sperm quality is time: both the time taken by an individual to deliver his sample to the laboratory, and the time that the samples remain in the laboratory for analysis. According to a previous study, the time from sample collection to transfer to the laboratory should not exceed 1 hour [5]. Sample incubation in the laboratory should also not take more than 2 hours [6]. In an andrology laboratory, semen samples are incubated before analysis or use in ART. This incubation can be done at room temperature (RT) or at 37°C, the latter of which is the usual incubation temperature [7]. The purpose of this incubation is the liquefaction process, which may take about 15 to 30 minutes. However, ejaculates may be incubated longer in the laboratory before analysis or use in ART.
Other specific parameters can be also assessed in order to assess sperm health. One of the main factors is the sperm DNA status, which can be checked for fragmentation, denaturation, or chromatin compression [8]. Sperm DNA integrity plays an important role in ART treatment cycles, and DNA damage has a considerable effect on clinical outcomes [9]. Studies have shown that there is a significant reverse relationship between sperm DNA damage and embryo formation, fetal growth, and pregnancy [10]. The most common cause of sperm DNA breakdown is oxidative stress [11]. In the male reproductive system, reactive oxygen species (ROS) are involved in many physiological processes, including capacity building, hyperactivity, acrosomal reaction, and fertilization process [12]. Spermatozoa are vulnerable to oxidative stress due to the presence of abundant unsaturated fatty acids on the surface of the plasma membrane and the lack of protective enzymes in the cytoplasm [13]. The presence of ROS produced by sperm and multinucleated leukocytes in semen can damage sperm DNA [14]. In addition, the mitochondria are another factor affecting sperm health. The main function of sperm mitochondria is to synthesize adenosine triphosphate (ATP) through oxidative phosphorylation. Although, the true contribution of mitochondrial-produced ATP in sperm is not fully understood, mitochondrial function is related to sperm quality. Mitochondrial status plays an important role due to its relationship with cell energy status and motility related to male fertility [15].
It should be noted that sperm cells are not able to fertilize an egg immediately after ejaculation. The acrosome reaction refers to the structural and metabolic changes during which sperm acquire the ability to fertilize an egg [16]. Studies have shown that damage to the acrosome reaction may lead to infertility [17]. The aim of this study was to assess the sperm parameters, ROS production, sperm mitochondria, sperm chromatin, and acrosomal reaction in samples of normozoospermic men at different time intervals (0, 1, 1.5, and 2 hours) after incubation at 37°C. To our knowledge, no previous studies have assessed the biological characteristics of spermatozoa at different time intervals using neat seminal samples.
Samples were collected from 25 normozoospermic men (aged 20–40 years) referred to the Yazd Reproductive Science Institute from August to October 2021. The samples were prepared in sterile containers by masturbation after 2–5 days of sexual abstinence. A consent form was signed by all participants. This study was approved by the Ethics Committee of Shahid Sadoughi University (IR.SSU.MEDICINE.REC.1400.132).
The semen samples were incubated at 37°C without washing or adding solution. Incubation was performed for four different time intervals: 0 (after liquefaction for 10–15 minutes), 1, 1.5, and 2 hours. Samples were evaluated for sperm parameters at each time interval.
Sperm analysis was performed according to the WHO guidelines [18]. To evaluate sperm motility, 10 μL of the sample was placed on the slide. A contrast phase microscope (Nikon, Tokyo, Japan) was used to assess 200 spermatozoa in five fields at ×200 magnification. Sperm motility was reported as the non-motile, non-progressive, and progressive motility percentages [18]. Sperm viability was assessed by eosin-Nigrosin staining. In this method, 10 μL of the sample was mixed with 10 μL of Eosin-Nigrosin. After 30 seconds, 10 μL of the mixture was placed on a slide and a smear was prepared. Then, 200 sperm cells were examined with a light microscope (×1,000 magnification). Dead sperm cells were stained pink or red, while viable sperm remained colorless [18]. Finally, the percentage of live spermatozoa was reported.
Diff-Quick staining kit (Dian Bio assay, Tehran, Iran) was used to evaluate sperm morphology. In this method, 10 μL of the sample was placed on a slide and a smear was prepared. After drying, the smear was fixed with 95% ethanol. The slide was then stained with eosin for 10 seconds and methylene blue for 5 seconds. The slides were finally rinsed with tap water. After slide drying, 200 sperm cells were evaluated by light microscopy (×1,000 magnification). The percentage of normal spermatozoa was reported [18].
A halo sperm kit (SDFA kit; Ideh Varzan Farda, Tehran, Iran) was used to assess the sperm chromatin condition. This method is not complicated and time-consuming, although it is economical [19]. In brief, 20 μL of low-melting agarose was combined with 50 μL of semen samples. Then, 20 μL of the mixture was placed on the precoated glass slide. A coverslip was placed on the drop to spread evenly over the slide. The slide was placed in the refrigerator at 4°C for 5 minutes. Next, the coverslip was slowly removed from the slide and the denaturing solution (A) was added. The slides were kept in a dark room at RT for 7 minutes. The lysing solution (B) was added to the slide and placed at RT for 15 minutes. The slide was washed with distilled water for 5 minutes. For dehydration, the slides were immersed in 70%, 90%, and 100% ethanol solutions for 2 minutes, respectively, and dried for 2 minutes at RT. First, the slides were stained with solution C for 75 seconds, then solution D for 3 minutes and finally solution E for 2 minutes. After that, the slides were washed with distilled water and 200 sperm cells were examined with bright-field microscopy (×1,000). The DNA fragmentation rate was evaluated according to halo formation around the sperm head. A medium or large halo around the sperm head indicated DNA without fragmentation, and the absence of a halo represented fragmented DNA [20]. The percentage of sperm with fragmented DNA (no halo) was reported.
For acrosome reaction evaluation, 10 μL of the sample was placed on a slide and a smear was prepared. After smear drying in air, the sample was fixed with 95% ethanol. Then, the slides were stained with 25 μL of fluorescein isothiocyanate-conjugated Pisum sativum agglutinin solution and incubated at 4°C for 1 hour. The slides were washed with distilled water and air-dried. Later, the slides were evaluated with a fluorescent microscope at 450–495 nm under ×400 magnification. The sperm with the acrosome reaction had a fluorescent band in the middle, while the rest of the parts did not have a fluorescent color, and more than half of the head of the sperm without the acrosome reaction was bright [21]. The percentage of spermatozoa with the acrosome reaction was reported.
The mitochondrial membrane potential (MMP) was evaluated using a JC1 kit (Cayman, Ann Arbor, MI, USA). First, 25 μL of the sample was mixed with 25 μL of JC-1 dye in a microtube and incubated in a CO2 incubator at 37°C for 15–30 minutes. After incubation, 100 μL of phosphate-buffered saline was added to the samples. Each sample was centrifuged at RT for 5 minutes at 400 ×g and then the supernatant was gently removed. Then, 10 μL of the sample was placed on a slide and a smear was prepared. After the smear was dried, 200 spermatozoa were assessed with a fluorescent microscope under ×1,000 magnification at 520–570 nm. Sperm cells with a red or shiny middle piece were considered to have high mitochondrial potential, or JC-1(+), and sperm cells with a green middle piece were considered to have low mitochondrial potential, or JC-1(–) [22]. The percentage of cells with high mitochondrial potential, denoted as JC-1(+), was reported.
The amount of malondialdehyde (MDA) in the samples was measured by a TPR kit (Teb Pazhoohan Razi, Tehran, Iran). First, 100 μL of the sample was poured into a microtube and 100 μL of the standard solution was added. Then, 100 μL of reagent (R4) and 200 μL of chromogen were added to the microtube. The microtube was first placed in a hot water bath for 1 hour, and then on ice for 10 minutes. The sample was finally centrifuged at 4°C for 15 minutes, and 200 μL of the samples were placed in the plate wells and inserted into the plate reader. The absorption rate was recorded by the device at 530–540 nm.
Data were analyzed in IBM SPSS ver. 26 (IBM Corp., Armonk, NY, USA). The Kolmogorov-Smirnov test was used to evaluate the normality of the data. Certain data was shown to be non-parametric distribution, the Friedman test was used to evaluate the trends. In addition, multiple comparisons among different times were performed, and the Bonferroni correction was used to adjust the statistical significance. A p-value <0.05 was considered to demonstrate a statistically significant difference in the Friedman test.
The results of different incubation times on sperm parameters are shown in Table 1. Briefly, after 1.5 hours and 2 hours, both progressive motility and normal morphology decreased compared to 0 hours and 1 hour. Furthermore, the percentage of non-motile sperm increased after 2 hours compared to 0 hours. No significant changes were observed in non-progressive motility (p=0.48) and viability (p=0.98) at any time intervals.
After incubation, sperm DNA fragmentation increased significantly (Figure 1). No significant changes were observed at 1 hour compared to 0 hours (median [interquartile range]: 19.5 [4] vs. 19 [4]), as well as at 1.5 hours compared to 1 hour (20 [5]). However, a significant increase in DNA fragmentation was observed at 2 hours compared to 0 hours and at 1 hour and at 1.5 hours versus 0 hours. The effects of incubation on the acrosome reaction are shown in Figure 2. No significant differences were observed at 0, 1, 1.5, or 2 hours.
As presented in Figure 3, no significant changes were noted after incubation in semen MDA levels. The effects of different incubation times on the MMP are presented in Figure 4. No significant difference in the MMP was observed at 1 hour compared with 0 hours (p=0.11) and at 1.5 hours versus 1 hour (median [interquartile range]: 79.5 [11] vs. 80.5 [10]). However, the sperm MMP decreased significantly at 2 hours compared to 0 and 1 hours and at 1.5 hours versus 0 hours (p<0.001).
The aim of this research was to investigate the most suitable time intervals for semen incubation in the laboratory for common and specific sperm parameters, such as DNA fragmentation, the acrosome reaction, MMP, and MDA production levels. The significant contribution of this research was its documentation of the effects of different time intervals on the semen preparation procedure, which is expected to help professionals choose the optimal conditions. The data showed that sperm motility changed at different time intervals. A decreasing trend was observed in the percentage of progressive motility after longer incubation periods. The percentage of progressive motile sperm decreased after 1.5 hours compared to the previous times. Moreover, when the incubation time exceeded 1.5 hours, the percentage of immotile spermatozoa in semen samples increased significantly. Sperm abnormal morphology, as well as DNA fragmentation, increased beyond 1.5 hours of incubation time. The MMP decreased remarkably after 1 hour of incubation time.
In the process of sperm preparation for different uses, especially in ART procedures, the incubation of semen at 37°C plays a crucial role [6]. Prolonged incubation of spermatozoa is performed in order to accommodate delays in oocyte preparation and pick-up, oocyte maturation, and rescue intracytoplasmic sperm injection (ICSI) [23]. Since the last decade, the assessment of spermatozoa incubation in laboratories has been a matter of debate. Although the influence of incubation time on conventional parameters of semen was investigated previously [24], specific factors, such as the acrosome reaction, DNA fragmentation, MMP, and MDA production levels, required more attention. Ouitrakul et al. [25] demonstrated that sperm motility decreased after 1 hour of incubation at RT, while the sperm viability was affected after 2 hours. The viability assessment in their study did not significantly change before 2 hours of incubation, which is in agreement with our findings. Furthermore, progressive motility decreased significantly after 2 hours, which is in agreement with the above study. Nematollahi et al. [26] showed that the percentage of progressive motile spermatozoa decreased in both the RT and 37°C groups compared to the fresh group (control). Furthermore, similar to our findings, more immotile spermatozoa were observed at 24 hours at 37°C than in the fresh group. In the current study, we observed that semen incubation induced morphological changes in sperm heads. Peer et al. [27] showed that the percentage of spermatozoa with vacuolated nuclei increased after 2 hours of incubation. Ahmed et al. [28] also reported that abnormal sperm morphology increased after 5 hours of incubation at 37°C. Our findings showed an increase in sperm abnormal morphology at 1.5 hours of incubation, which is similar to the previous studies. Another adverse effect of incubation is DNA fragmentation. Our findings were similar to those of Nabi et al. [6], who reported a significant increase in DNA fragmentation after 2 hours of incubation. Alvarez Sedo et al. [29] also showed that DNA fragmentation of semen prepared by the swim-up method significantly increased after 4 hours of incubation, which is in agreement with this study. The production of ATP is one of the main functions of mitochondria, which are required primarily for sperm motility and cellular events involved in capacity, hyperactivity, and the acrosome reaction. The MMP decreased notably after 1 hour of incubation. Zhu et al. [30] showed that incubation of sperm after 6 hours in a low-glucose solution led to reductions in both ATP levels and the MMP. An acrosomal cap covers about 40% to 70% of the anterior region of the sperm nucleus. Acrosomes contain hydrolyzing enzymes that are released to digest oocytes during the acrosomal reaction. The hyperactive conditions related to the acrosome reaction result in the penetration of sperm into the zona pellucida and interactions with the oocyte. In the current study, the acrosome reaction did not change significantly at different time intervals. By increasing time intervals, adverse results may be encountered. It has been suggested that sperm DNA fragmentation may be related to apoptosis events and oxidative stress. Sperm cells are very vulnerable to damage induced by ROS, because the sperm membrane contains large amounts of polyunsaturated fatty acids. MDA, as an indicator of oxidative damage, is an end product of lipid peroxidation of the sperm membrane, which can affect sperm structure, and function.
At infertility centers, to perform ART cycles, sperm cells should be prepared and kept for approximately 2 hours in a 37°C incubator or at RT before microinjections, which may have destructive effects on clinical outcomes. In this regard, Pujol et al. reported that the rate of clinical pregnancy decreased due to a long time period between oocyte pick-up and ICSI [31]. Altogether, this study suggests that the ideal incubation time to reduce the adverse effects on vital semen parameters should be less than 1.5 hours, which may contribute to better clinical outcomes for infertile individuals. This information may also encourage patients to deliver their samples to the laboratory within the optimal period of time. Therefore, in andrology laboratories, paying attention to the incubation time in semen preparation protocols and ART performance is a noteworthy issue.
The incubation of normozoospermic samples before use in ART should be less than 1.5 hours to minimize the possible destructive effects of a prolonged incubation time on specific sperm parameters. Although sperm with prolonged incubation can fertilize oocytes, the clinical outcomes may be altered.
Figure 1.
Effects of different incubation times on sperm DNA integrity. There were significant changes at 1.5 hours versus 0 hours (p<0.001) and 2 hours (p<0.001). Furthermore, at 1 hour versus 2 hours (p<0.001), a significant increase in DNA fragmentation was observed.

Figure 2.
Effect of different incubation times on the acrosome reaction (AR) in sperm. No significant differences were found in the AR according to time (p=0.34).
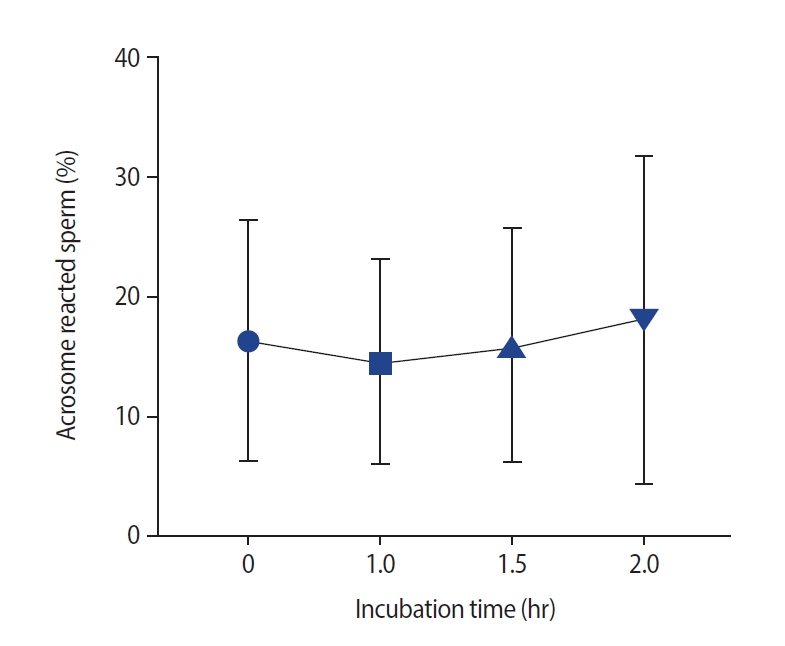
Figure 3.
Effect of different incubation times on semen malonaldehyde (MDA) levels. No significant correlation was found between incubation time and semen MDA levels (p=0.98).
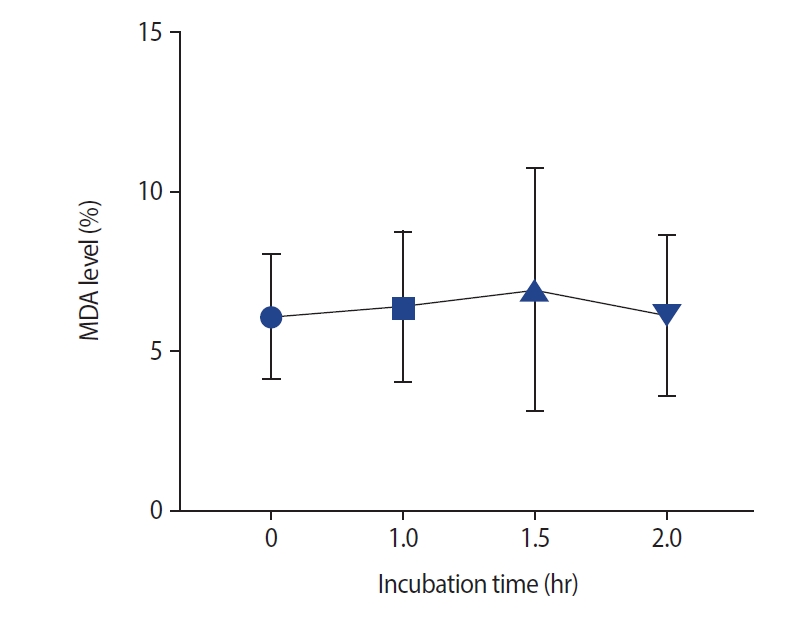
Figure 4.
Effect of different incubation times on the mitochondrial membrane potential (MMP), as measured using the JC-1 assay. The mitochondrial membrane temperature was reduced with long incubation of spermatozoa. Significant changes were observed at 1.5 hours versus 0 hours (p<0.001), as well as at 2 hours versus 0 hours (p<0.001) and at 2 hours versus 1 hour (p<0.001).
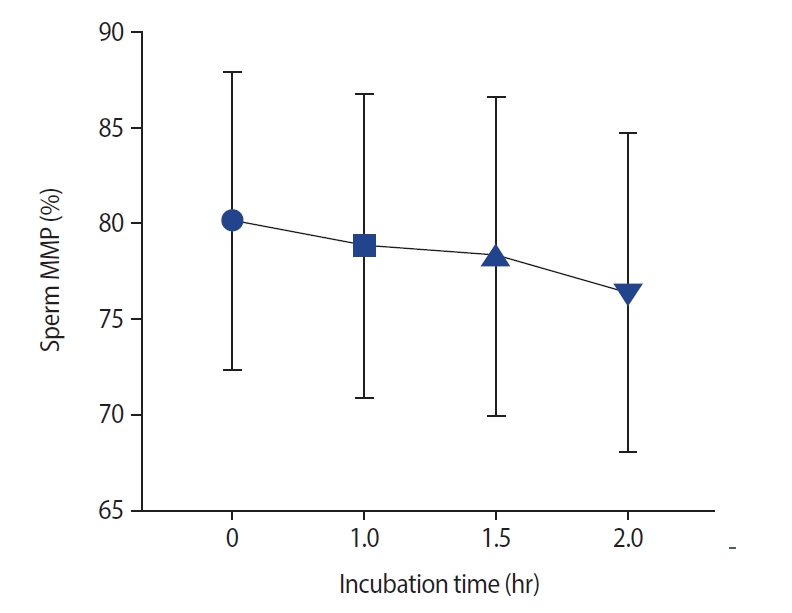
Table 1.
Effect of different incubation times on sperm parameters
| Sperm parameter |
Incubation time (hr) |
p-value | |||
|---|---|---|---|---|---|
| 0 | 1 | 1.5 | 2 | ||
| Progressive motility (%) | 55.14±12.03a) | 52.82±9.59b) | 50.45±10.25 | 48.45±10.22 | <0.001e) |
| Median (IQR) | 20 (17) | 50 (15) | 48 (14) | 49 (13) | |
| Non-progressive motility (%) | 2.86±2.35 | 2.86±2.25 | 2.64±2.77 | 2.82±2.73 | 0.997 |
| Median (IQR) | 2 (4) | 2 (3) | 2 (3) | 2 (5) | |
| Non-motility (%) | 42.50±11.65c) | 44.32±9.48 | 46.41±10.34 | 48.68±10.22 | <0.001e) |
| Median (IQR) | 46 (17) | 48.5 (12) | 48.5 (16) | 50.5 (12) | |
| Viability (%) | 80.91±11.50 | 80.05±13.81 | 78.95±15.52 | 79.41±13.72 | 0.986 |
| Median (IQR) | 85.5 (15) | 85.5 (17) | 85 (18) | 84.5 (17) | |
| Normal morphology (%) | 7.55±5.51d) | 7.32±6.46 | 6.00±5.72 | 5.68±6.21 | <0.001e) |
| Median (IQR) | 5 (4) | 4.5 (5) | 4.5 (4) | 3.5 (5) | |
References
1. Rowe PJ, Comhaire FH, Hargreave TB, Mahmoud AM. WHO manual for the standardized investigation and diagnosis of the infertile male. Cambridge: Cambridge university press; 2000.
2. Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol 2015;13:37.




3. Gnoth C, Godehardt E, Frank-Herrmann P, Friol K, Tigges J, Freundl G. Definition and prevalence of subfertility and infertility. Hum Reprod 2005;20:1144-7.


4. Jensen CF, Khan O, Nagras ZG, Sonksen J, Fode M, Ostergren PB, et al. Male infertility problems of patients with strict sperm morphology between 5-14% may be missed with the current WHO guidelines. Scand J Urol 2018;52:427-31.


5. Sunder M, Leslie SW. Semen analysis [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 [cited 2022 Sep 19]. Available from: https://pubmed.ncbi.nlm.nih.gov/33232039/
6. Nabi A, Khalili MA, Halvaei I, Roodbari F. Prolonged incubation of processed human spermatozoa will increase DNA fragmentation. Andrologia 2014;46:374-9.


7. van der Westerlaken L, Naaktgeboren N, Verburg H, Dieben S, Helmerhorst FM. Conventional in vitro fertilization versus intracytoplasmic sperm injection in patients with borderline semen: a randomized study using sibling oocytes. Fertil Steril 2006;85:395-400.


8. Mangoli E, Khalili MA, Talebi AR, Ghasemi-Esmailabad S, Hosseini A. Is there any correlation between sperm parameters and chromatin quality with embryo morphokinetics in patients with male infertility? Andrologia 2018;50:e12997.



9. Yang H, Li G, Jin H, Guo Y, Sun Y. The effect of sperm DNA fragmentation index on assisted reproductive technology outcomes and its relationship with semen parameters and lifestyle. Transl Androl Urol 2019;8:356-65.



10. Zhu XB, Chen Q, Fan WM, Niu ZH, Xu BF, Zhang AJ. Sperm DNA fragmentation in Chinese couples with unexplained recurrent pregnancy loss. Asian J Androl 2020;22:296-301.


11. Ritchie C, Ko EY. Oxidative stress in the pathophysiology of male infertility. Andrologia 2021;53:e13581.



12. Sabeti P, Pourmasumi S, Rahiminia T, Akyash F, Talebi AR. Etiologies of sperm oxidative stress. Int J Reprod Biomed 2016;14:231-40.




13. Talebi AR, Khalili MA, Vahidi S, Ghasemzadeh J, Tabibnejad N. Sperm chromatin condensation, DNA integrity, and apoptosis in men with spinal cord injury. J Spinal Cord Med 2013;36:140-6.



14. Bashiri Z, Amidi F, Amiri I, Zandieh Z, Maki CB, Mohammadi F, et al. Male factors: the role of sperm in preimplantation embryo quality. Reprod Sci 2021;28:1788-811.



15. Amaral A, Lourenco B, Marques M, Ramalho-Santos J. Mitochondria functionality and sperm quality. Reproduction 2013;146:R163-74.


16. Ickowicz D, Finkelstein M, Breitbart H. Mechanism of sperm capacitation and the acrosome reaction: role of protein kinases. Asian J Androl 2012;14:816-21.



17. El-Taieb MA, Ali MA, Nada EA. Oxidative stress and acrosomal morphology: a cause of infertility in patients with normal semen parameters. Middle East Fertil Soc J 2015;20:79-85.

18. World Health Organization. WHO laboratory manual for the examination and processing of human semen. Geneva: World Health Organization; 2010.
19. Anbari F, Halvaei I, Nabi A, Ghazali S, Khalili MA, Johansson L. The quality of sperm preparation medium affects the motility, viability, and DNA integrity of human spermatozoa. J Hum Reprod Sci 2016;9:254-8.



20. Nabi A, Khalili MA, Fesahat F, Talebi A, Ghasemi-Esmailabad S. Pentoxifylline increase sperm motility in devitrified spermatozoa from asthenozoospermic patient without damage chromatin and DNA integrity. Cryobiology 2017;76:59-64.


21. Esteves SC, Sharma RK, Thomas AJ Jr, Agarwal A. Evaluation of acrosomal status and sperm viability in fresh and cryopreserved specimens by the use of fluorescent peanut agglutinin lectin in conjunction with hypo-osmotic swelling test. Int Braz J Urol 2007;33:364-76.


22. Sivandzade F, Bhalerao A, Cucullo L. Analysis of the mitochondrial membrane potential using the cationic JC-1 dye as a sensitive fluorescent probe. Bio Protoc 2019;9:e3128.



23. Karimi Zarchi M, Maleki B, Dehghani Ashkezari M, Motamed Zadeh L, Agha-Rahimi A. The effects of in vitro incubation of asthenoteratozoospermic semen after density gradient centrifugation at room temperature and 37°C on sperm parameters, chromatin quality and DNA fragmentation in a short time period. J Reprod Infertil 2020;21:275-82.


24. Thijssen A, Klerkx E, Huyser C, Bosmans E, Campo R, Ombelet W. Influence of temperature and sperm preparation on the quality of spermatozoa. Reprod Biomed Online 2014;28:436-42.


25. Ouitrakul S, Sukprasert M, Treetampinich C, Choktanasiri W, Vallibhakara SA, Satirapod C. The effect of different timing after ejaculation on sperm motility and viability in semen analysis at room temperature. J Med Assoc Thai 2018;101:26-32.
26. Nematollahi S, Mehdizadeh M, Hosseini S, Kashanian M, Amjadi FS, Salehi M. DNA integrity and methylation changes of mouse spermatozoa following prolonged incubation. Andrologia 2019;51:e13276.



27. Peer S, Eltes F, Berkovitz A, Yehuda R, Itsykson P, Bartoov B. Is fine morphology of the human sperm nuclei affected by in vitro incubation at 37°C? Fertil Steril 2007;88:1589-94.

28. Ahmed I, Abdelateef S, Laqqan M, Amor H, Abdel-Lah MA, Hammadeh ME. Influence of extended incubation time on human sperm chromatin condensation, sperm DNA strand breaks and their effect on fertilisation rate. Andrologia 2018;50:e12960.


29. Alvarez Sedo C, Bilinski M, Lorenzi D, Uriondo H, Noblia F, Longobucco V, et al. Effect of sperm DNA fragmentation on embryo development: clinical and biological aspects. JBRA Assist Reprod 2017;21:343-50.


- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 1,732 View
- 133 Download
- Related articles in Clin Exp Reprod Med
-
Comparison among the sperm preparation methods on the human spermatozoa.1993 August;20(2)






