 |
 |
- Search
| Clin Exp Reprod Med > Volume 49(4); 2022 > Article |
|
Abstract
Objective
This research investigated the effects of human chorionic gonadotropin (HCG)-producing peripheral blood mononuclear cells (PBMCs) on the implantation rate and embryo attachment in mice.
Methods
In this experimental study, a DNA fragment of the HCG gene was cloned into an expression vector, which was transfected into PBMCs. The concentration of the produced HCG was measured using enzyme-linked immunosorbent assay. Embryo attachment was investigated on the co-cultured endometrial cells and PBMCs in vitro. As an in vivo experiment, intrauterine administration of PBMCs was done in plaque-positive female mice. Studied mice were distributed into five groups: control, embryo implantation dysfunction (EID), EID with produced HCG, EID with PBMCs, and EID with HCG-producing PBMCs. Uterine horns were excised to characterize the number of implantation sites and pregnancy rate on day 7.5 post-coitum. During an implantation window, the mRNA expression of genes was evaluated using real-time polymerase chain reaction.
Results
DNA fragments were cloned between the BamHI and EcoRI sites in the vector. About 465 pg/mL of HCG was produced in the transfected PBMCs. The attachment rate, pregnancy rate, and the number of implantation sites were substantially higher in the HCG-producing PBMCs group than in the other groups. Significantly elevated expression of the target genes was observed in the EID with HCG-producing PBMCs group.
Despite improvements in assisted reproductive technology, the overall fertilization rate in infertile patients referred to infertility centers remains low [1,2]. The failure of embryo implantation in the uterus is a critical factor in patients with infertility. The secretion of steroid hormones prepares the endometrium for embryo receptivity and implantation; these hormones stimulate structural and functional changes in the endometrium and ready the uterus for implantation. However, steroid hormone supplements administered to women with infertility problems do not have a considerable effect on endometrial function [3,4]. Studies have shown that local immune cells at the implantation site have an important role in the success of implantation. Peripheral blood mononuclear cells (PBMCs) consist of lymphocytes (B and T cells) and monocytes. PBMCs produce various cytokines, such as interleukin (IL)-1, tumor necrosis factor α (TNF-α), and IL-6; these cytokines have a positive impact on the endometrial receptivity of embryos in humans [5,6]. In addition, PBMCs are involved in hemochorial placentation by regulating initiation, controlling invasion, and adjusting immune tolerance in embryo implantation [5]. Hence, immune therapy can be an effective approach for the enhancement of endometrial receptivity [5,6]. Human chorionic gonadotropin (HCG), as a steroid hormone, can initialize arrays of molecular messages between the decidua and blastocyst. HCG promotes progesterone production by corpus luteal cells and also boosts angiogenesis in the uterine vasculature through the activation of endometrial cells [7]. Yoshioka et al. [8] found that intrauterine injection of autologous PBMCs activated with HCG could raise implantation, pregnancy, and live birth rates in infertility patients. Pourmoghadam et al. [9] demonstrated that intrauterine administration of autologous HCG-activated PBMCs in women with recurrent implantation failure (RIF) who had a low Th-17/Treg cell ratio significantly increased as compared to the control group. Furthermore, Okitsu et al. [10] showed that the intrauterine injection of autologous PBMCs beneficially boosted embryo implantation in patients who received frozen-thawed embryo transfer after three or more repeated in vitro fertilization (IVF) failures. Another study by Li et al. [11] showed that the intrauterine administration of HCG-activated autologous human PBMCs promoted the live birth rate in frozen/thawed embryo transfer cycles of patients with RIF.
The numerous changes that occur in the endometrium, wherever PBMCs impact endometrial receptivity, may explain these improvements [5,12]. Consequently, we conclude that peripheral immune cells receive signals of the conceptus in the initial stage of pregnancy that activate PBMCs. Activated PBMCs are useful in the expression and production of immune molecules at the implantation site. Immune molecules adjust endometrial receptivity in the maternal-embryonic interface for implantation. All previous studies on this topic have focused on the role of HCG-activated PBMCs by incubating PBMCs with HCG in embryo implantation. We designed this study from a new perspective, exploring the use of HCG-producing PBMCs to increase embryo implantation. Therefore, our objective in the current study was to assess the impact of HCG-producing PBMCs on embryo attachment, the number of implantation sites, and the pregnancy rate in the mice uterus. Furthermore, we investigated the effects of HCG-producing PBMC on the gene expression level of Il-6, Il-1β, leukemia inhibitory factor (Lif), vascular endothelial growth factor (Vegf), matrix metalloproteinase 9 (Mmp9), Janus kinase 2 (Jak2), and signal transducer and activator of transcription 3 (Stat3) genes in the uterus and the underlying mechanisms.
All materials were prepared from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise specified. This study was approved by the Research and Ethics Committee of Shahid Beheshti University of Medical Sciences with the IR.SBMU.RETECH.REC.1396.1297 design code (Tehran, Iran). B6D2F1 (C57BL/6×DBA2) mice (Royan Institute, Tehran, Iran) were used in this experiment. B6D2F1 mice are hybrid and are better for reproductive studies because they have a higher pregnancy rate and release more suitable oocytes. The mice were maintained in standard conditions of a 12-hour light/12-hour dark cycle, at temperatures of 22°C–28 ̊C temperature. Figure 1 provides an outline of the research workflow.
The HCG gene sequence was obtained from the Reference Sequence (RefSeq) database of the National Center for Biotechnology Information (NCBI; www.ncbi.nlm.nih.gov). The HCG gene was then constructed in a pGH cloning vector (Bioneer, Daejeon, Korea). Plasmid expressing green fluorescent protein-N1 (pEGFP-N1) as an expression vector was selected for insertion of the synthetic HCG gene. First, the pGH and pEGFP-N1 vectors were digested with BamHI and EcoRI enzymes (Takara, Dalian, China). An agarose gel extraction kit (Qiagen, Hilden, Germany) was used for DNA extraction and purification of the HCG DNA. The purified DNA was inserted into the pEGFP-N1 vector by T4 DNA ligase, and the ligation reaction was transformed into an Escherichia coli top 10 competent cell. Clones of bacteria were assayed using colony polymerase chain reaction (PCR). Positive clones were confirmed by digestive enzymes (BamHI and EcoRI) along with DNA sequencing (sanger sequencing; ABI3500 Genetic Analyzer, Foster City, CA, USA).
PBMCs were separated from non-pregnant mice by density gradient centrifugation, using Ficoll-Paque (St. Louis, MO, USA), collected and washed with phosphate-buffered saline (PBS) and then re-suspended in pre-warmed standard Roswell Park Memorial Institute RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and 100 U/mL penicillin/streptomycin. pEGFP-N1-HCG and pEGFP-N1 (empty vector) vectors were used for transfection of the PBMCs. Transfection of PBMCs with a pEGFP-N1 vector containing the HCG gene was done according to our previous study procedure [13]. PBMCs were also transfected with the empty pEGFP-N1 vector (empty vector).
The concentration of produced HCG in the transfected PBMCs was estimated by the enzyme-linked immunosorbent assay (ELISA) method. The cell culture supernatants were harvested, and the amount of HCG (at a cell density of 1×106 cells/mL) was determined by a commercially available ELISA kit (Biocompare, South San Francisco, CA, USA) every 24 hours.
Epithelial and stromal cells were isolated from the mouse endometrium as follows: the vasectomized male BDF1 mice were mated with female mice from the same strain. Vaginal plug-positive mice were considered pseudo-pregnant. The pseudo-pregnant mice were killed by cervical dislocation, and the uterus was removed and washed with PBS that contained 300 U/mL penicillin/streptomycin; after that, adipose and connective tissues were separated, and the uterine horns were maintained in pre-warmed medium supplemented with collagenase for 1 hour in a humidified atmosphere of 5% CO2 and 37°C. Later the uterine horns were scraped and placed in digestion media containing collagenase and dispase enzymes for 2–3 hours in a humidified atmosphere at 37°C with 5% CO2. During this time, the tissue pieces were slowly mixed once every 30 minutes; afterwards, the cell suspension was precipitated by centrifugation at 470 ×g for 5 minutes. The supernatant was transferred to 25 cm2 tissue culture flasks with Dulbecco̕ s Modified Eagle Medium/Nutrient Mixture F12 plus 15% FBS (DMEM/F12) and maintained in a humidified incubator with a 5% CO2 atmosphere at 37°C for 72 hours.
For co-culture of isolated endometrial cells with PBMCs, passage-2 primary endometrial cells (1×106 cells/mL) were added in DMEM/F12 medium supplemented with 15% FBS, 100 nmol/L beta-estradiol, and 10 nmol/L progesterone and placed in a humidified incubator with a 5% CO2 atmosphere at 37°C for 3 days (72 hours). Endometrial cells and PBMCs were prepared at a 1:1 ratio for the co-culture cells. Isolated endometrial cells without PBMCs were applied as the control group.
On the IVF day, male BDF1 mice were killed by cervical dislocation. Spermatozoa were separated from the cauda epididymis and vasa deferentia. Sperm suspensions were isolated and maintained in a human tubal fluid medium (HTF) containing 4 mg/mL bovine serum albumin (BSA) and incubated for 45 minutes at 37°C. Subsequently, the 6- to 8-week-old female BDF1 mice were superovulated by intraperitoneal injections of 10 IU pregnant mare serum gonadotropin (PMSG) and after 50 h, to obtain relatively large numbers of oocytes 10 IU hCG were injected intraperitoneally. Next matured MII oocytes were separated from the ampulla of the oviducts of female mice and placed in drops of 100 µL HTF medium supplemented with 4 mg/mL BSA. Immediately, approximately 1×106 sperm/mL was added to the HTF drops and incubated under the above incubation conditions for 6 hours. After 6 hours, the hypothetical fertilized zygotes were transferred to the potassium simplex-optimized medium containing 4% BSA. One day after IVF, the number of two-cell embryos was counted to assess the fertilization rate by an optical microscope. Four days after IVF, the oocytes were ultimately investigated for blastocyst formation [14].
The co-cultured cells (endometrial cells with PBMCs) were seeded in DMEM/F12 medium and incubated for 72 hours. Next, zona-free expanded blastocysts, obtained using Acid Tyrode’s solution (n=10), were placed on the cultured cell plates and incubated for 72 hours. The cell culture plates were divided into three groups (endometrial cells, co-culture of endometrial cells with PBMCs, and co-culture of endometrial cells with HCG-producing PBMCs [transfected PBMCs]) and were swirled in a circular motion once every 24 hours, and the numbers of embryos that stayed at the same location were counted for the blastocyst attachment rate. Unattached blastocysts were floated in the medium.
To assess the role of HCG-producing PBMCs on implantation and pregnancy rate, embryo implantation dysfunctional (EID) female B6D2F1 mice models were created using a small dose of mifepristone (0.08 mg/0.1 mL, soluble in propanediol). Mice were randomly divided into three groups: control (without PBMC and mifepristone), EID, EID with PBMCs, EID with HCG-producing PBMCs, and EID with produced HCG. The super-ovulated mice were mated with male mice at a ratio of 2:1. The vaginal plug-positive mice were selected, since the vaginal plug is a symptom of mating, and pregnant mice were identified on the day the vaginal plug was detected, considered as day 0.5 post-coitum. At 9:00 am on day 3.5 post-coitum, 0.1 mL of mifepristone was injected subcutaneously to all groups except the control group [15].
PBMCs and HCG-producing PBMCs were prepared in medium culture as described above. On day 1.5 post-coitum, mice were anesthetized and 5 µL (106 cells/mL) of HCG-producing PBMCs were injected surgically into the cranial part of each uterine horn using a micro-syringe in the EID with HCG-producing PBMCs group. The EID with PBMCs and EID with HCG groups also received 5 µL of PBMCs (1×106 cells/mL) and 450 pg/mL of produced HCG, respectively. An empty culture medium was used in the control and EID groups. On day 3.5 post-coitum at 22:00, some of the studied mice in each group were sacrificed. Next, the uterus was isolated and cryopreserved at –70°C for a real-time PCR test, while the other studied mice were sacrificed on day 7.5 post-coitum and the bifurcated uterus was removed. The numbers of pregnant mice and implantation sites were assessed in each group.
Total RNA was extracted from endometrial tissues using a commercial mini kit (GeneAll Hybrid-R RNA Purification Kit, Seoul, Korea) according to the manufacturer’s instructions. The quantity and quality of the RNA were confirmed by spectrophotometry (Eppendorf, Hamburg, Germany) and gel electrophoresis. Samples were reverse-transcribed by the protocol described in previous studies [13]. Briefly, 3 μL of random hexamers, 5 μL of nuclease-free water, and 4 μL of RNA were added to each microtube. The microtubes were incubated in a GeneMate (Fgeng02FT, Saint Paul, Minnesota, USA) series thermal cycler for 5 minutes at 75°C. The tubes were held on ice and cDNAs were synthesized using 5×reverse-transcriptase buffer, 200 U reverse transcriptase (RT) enzyme, 10 mmol/L dNTP, and 10 U RNase inhibitor. The reverse transcription reaction was carried out as follows: 25°C for 10 minutes, 37°C for 15 minutes, 42°C for 45 minutes, and 72°C for 10 minutes. DNA Master SYBR Green I Mix (Yekta Tajhiz, Tehran, Iran) was used for the quantitative real-time PCR (qRT-PCR) reactions for Il-6, Il-1β, Lif, Vegf, Mmp9, Jak2, and Stat3 according to the manufacturer’s recommendations. Amplification was performed in the following conditions: 95°C for 2 minutes, 95°C for 5 seconds, 60°C for 30 seconds, 72°C for 10 seconds, and 40 cycles of extension. Reverse transcription polymerase chain reaction (RT-PCR) was carried out in a Step One instrument (Applied Biosystems, Waltham, MA, USA) with beta-2-microglobulin (beta-2m) as the reference gene. Relative expression was estimated by the 2−∆∆CT method [16]. The primer information is listed in Table 1.
Data analysis was carried out with IBM SPSS ver. 20 (IBM Corp., Armonk, NY, USA). One-way analysis of variance and the Tukey test were used to calculate the rate of blastocyst attachment and implantation sites. REST 2009 Software (Qiagen, Hilden, Germany) was used to analyze RT-PCR data. A p-value <0.05 indicated statistical significance.
The accuracy of cloned HCG was checked by colony PCR, enzymatic digestion, and sequencing. Colony PCR was done with universal PE primers F: 5̕̕̕̕̕̕̕ CGCAAATGGGCGGTAGGCGTG 3̕ and R: 5̕̕̕̕̕̕̕ GGCCCGTTTACGTCGCCGTCC 3̕. A PCR product of approximately 670 bp proved the correctness of cloning in the expression vector (Figure 2A). The target insert band (560 bp) was determined in the enzyme digestion in Figure 2B, after double digestion with the BamHI and EcoRI enzymes.
PBMCs were transfected with a recombinant vector using an electroporation procedure. Protein production observed in transfected PBMCs was high at 48 hours after transfection, and the highest concentration of produced HCG was 465 pg/106 cells in transfected PBMCs (Figure 2C).
The microscopic examination of isolated endometrial cells showed that the epithelial cells were cuboidal to columnar and the stromal cells were spindle-shaped. Table 2 presents the developmental competence rate in IVF; approximately 84% of embryos developed into blastocysts. The blastocysts (no zona pellucida) were attached and migrated to the co-cultured cells within 24–72 hours, and the attachment and migration of the blastocysts are shown in Figure 3A-C. The blastocyst attachment rate was calculated as 82.5%±2.4% in the transfected PBMCs (HCG-producing PBMC) group, 59.5%±0.74% in the PBMCs group, 35%±1.92% in the endometrial cells with produced HCG group, and 26.11%±1.4% in the control group (endometrial cells without PBMCs and HCG). The results showed that the percentage of attached blastocysts was significantly higher in the transfected PBMCs group compared with the other groups (Figure 3D).
To determine the uterine receptivity, the pregnancy rate and the number of implantation sites were investigated on day 7.5 post-coitum. The pregnancy rate was lower in the EID group (33.33%) than in the control group (88.8%) (p=0.012). The pregnancy rates in the EID with produced HCG (77.7%) (p=0.038), EID with PBMCs (77.7%) (p=0.031), and EID with HCG-producing PBMCs (88.8%) (p=0.021) groups were higher than in the EID group (Figure 4A). The number of implantation sites in the EID group (1.66±1.52) was also lower compared to the control group (12.33±5.1) (p=0.014). After the intrauterine administration of produced HCG and PBMCs, the number of implantation sites was higher than in the EID group. Specifically, the numbers of implantation sites in EID with produced HCG group and EID with PBMCs were 6.0±1.0 (p=0.04) and 8.3±1.52 (p=0.032), respectively. The EID with HCG-producing PBMCs group showed a remarkably higher implantation site number (14.33±2.51) (p=0.001) than the EID group. Examples of the implantation sites of pregnant mice in each group are shown in Figure 4B-F on day 7.5 post-coitum.
The expression levels of Mmp-9, Lif, Jak2, Stat3, Vegf, Il-β, and Il-6, normalized by the level of beta-2m expression, were assessed in the endometrial tissues. The qRT-PCR analysis showed that Mmp-9, Lif, Jak2, Stat3, Vegf, Il-β, and Il-6 mRNA was upregulated in the endometrium of mice during the implantation window in the control group, EID, EID with HCG, EID with PBMCs, and EID with HCG-producing PBMCs (EID+recombinant vector) groups. Compared with the EID group, Lif, Jak2, Stat3, Vegf, Il-β, and Il-6 mRNA expression levels in the control group were significantly higher. The mRNA expression levels of genes were higher in the EID with produced HCG group, EID with PBMCs, and EID with HCG-producing PBMCs (EID+recombinant vector) groups than in the control group (Figure 5). The assessment of gene expression with real-time PCR was repeated three times.
The importance of implantation as a crucial process in reproduction has been proven, and one of the main reasons for the failure of embryo implantation in RIF patients is that the embryo does not implant well in the uterus [1,2]. In the maternal-fetal interface at the site of implantation, the presence of immune-related cells plays an important role in the creation of immune tolerance and moderate inflammatory response during implantation [17]. Our result showed that uterine administration of HCG-producing PBMCs had positive impacts on embryo attachment, blastocyst implantation in the uterus, and pregnancy rates. Also, mRNA levels of Mmp-9, Lif, Jak2, Stat3, Vegf, Il-β, and Il-6 were higher in the HCG-producing PBMCs group than in the PBMCs group. The abnormal immune environment of the endometrium is involved in improper embryo attachment [6]. Researchers have shown that 80% of patients with prior implantation failures had an imbalance of the immune system in the uterus. This issue could be a reason for the poor receptivity and attachment of the embryo into the endometrium [18]. It has been revealed that immune therapy such as lymphocyte therapy [19] or intravenous immunoglobulin [20] influences the development of pregnancy in RIF patients. Intrauterine injection of HCG-exposed PBMCs before transferring the embryo into the uterus has produced better pregnancy outcomes in RIF patients [11]. We found that intrauterine infusion of HCG-producing PBMCs could effectively enhance the number of implanted embryos in EID mice. It was also discovered that the attachment of blastocysts onto endometrial cells significantly increased in the presence of HCG-producing PBMCs. To investigate and determine the specific mechanism of HCG-producing PBMCs in the EID mice, we used qRT-PCR to examine the expression of the IL-1β, IL-6, Lif, Vegf, Mmp-9, Jak2, and Stat3 genes, which are involved in regulating embryo implantation. Our results showed that the presence of PBMCs and produced HCG upregulated the mRNA expression of inflammatory cytokines such as IL-1β and IL-6, and positively improved endometrial receptivity in the EID mice. PBMCs are engaged in the production of inflammatory cytokines, such as IL-1β and IL-6 [15]. A study in baboons showed that intrauterine injection of IL-1β and chorionic gonadotropin induced changes in the endometrium that mimicked the early events in pregnancy [15,21]. The current study demonstrated that HCG-producing PBMCs stimulated the production of IL-1β and IL-6, and subsequently showed improvement in embryo implantation and endometrial receptivity. Inflammatory cytokines such as IL-1β and TNF-α noticeably influence the production and secretion of Mmp-9 and Mmp-2, which increase the capability of human embryo invasion into the uterus [22,23]. Our data displayed that Mmp-9 was downregulated in the EID group compared with the control group, but it was upregulated with PBMCs and HCG therapy. One member of the IL-6 family is LIF, a cytokine associated with the Th2 response, which leads to the production of IL-4 [24]. LIF regulates numerous activities, including endometrium preparation for implantation, decidualization, growth, and development of the embryo, interaction between uterus and blastocyst, attachment of trophoblast into the endometrium, and the regulation of immunity involved in pregnancy [25]. The vital role of LIF has led to suggestions that LIF could be used as a biomarker for the assessment of endometrial receptivity [26]. The binding of LIF to its receptor activates the JAK/STAT signaling cascade. Activation of the JAK/STAT pathway is involved in regulating several biological responses such as cell growth, differentiation, longevity, and migration. Stimulating Stat-3 through LIF affects the expression of various genes. The expression of these genes controls different actions, for example cytokines and signaling (IL-6, OSMR, SOCS3, and JUNB), adhesion (CECAM1, PDPN, and ITGB3), invasion (PAPPA, caspase 1, SER PINB3, TIMP1, TIMP2, and TIMP3), and angiogenesis (ID1, ICAM1, EDIL3, and CCL2) [27]. We observed that HCG-producing PBMCs upregulated the expression of LIF and the Jak2/Stat3 genes. This suggests that intrauterine injection of these cells has a positive effect on uterine receptivity. VEGF, as a vascular permeability factor, regulates the proliferation of endothelial cells at the implantation site [28]. VEGF in rodents leads to permeability and uterine edema, and inhibition of VEGF is implicated in embryo development and implantation outcome [29]. Real-time gene expression analysis showed that during the implantation window, the expression level of Vegf was significantly upregulated in mice under conditions of EID with HCG-producing PBMCs. Our results showed that infusion of HCG-producing PBMCs into the uterus enhanced endometrial receptivity in mice; this increase in uterine receptivity may be due to improved physiological endometrial secretory changes or surface modification of expression molecules in endometrial cells [3].
Progesterone is one of the main factors in embryo implantation, and studies have indicated that the stimulation of progesterone production by luteal cells is mediated by PBMCs [4]. According to the evidence provided by previous studies, PBMCs receive information about the presence of the embryo at the sites of embryo implantation at the beginning of pregnancy. Next, PBMCs transfer information through the blood circulation into the ovaries and then regulate the function of the corpus luteum during pregnancy [30,31]. Therefore, PBMCs indirectly enable the embryo to be implanted in the uterus in keeping with HCG [32]. In pregnant women, PBMCs induce embryo invasion and also are directly involved in embryo outgrowth [32]. These results imply that PBMCs are very effective at indirectly regulating ovarian function and using directly inducing embryo invasion in early pregnancy [12,32]. A study showed that the spreading and invasion of mouse embryos were not affected by PBMCs and HCG; however, it was also observed that the presence of activated PBMCs with HCG in the uterus promoted the spreading and invasion of the embryos. This difference in outcomes may be related to a lack of chorionic gonadotropin production at the implantation site of the mouse embryos [32]. One of the limitations of our study was the different immunological environments of individual mice. Therefore, the use of this method in some people may not show a positive effect due to immunological dysfunction. Furthermore, our study did not keep track of the embryos for assessment of the live birth rate. Our results provide theoretical support for the potential clinical application of PBMCs and HCG simultaneously in patients with RIF. The effect of using expression vectors on human cells must be investigated, and precautions for clinical use related to the insertion and expression of the vector must be carefully studied. In conclusion, this study has shown that the HCG-producing PBMCs enhanced the number of implantation sites and the rate of embryo attachment in the endometrial cells. Alteration in cytokine expression could be a possible cause of the high embryo attachment rate, pregnancy rate, and the number of implantation sites. The results of our study show that it is possible that HCG-producing PBMCs could be used for patients suffering from RIF.
Acknowledgments
This work was performed at the Cellular and Molecular Biology Research Center of Shahid Beheshti University of Medical Sciences (Tehran, Iran). It was taken from a Ph.D. thesis by Delsuz Rezaee.
Figure 1.
Outline of research workflow. The HCG gene sequence was obtained from National Center for Biotechnology Information (NCBI) database, and synthesized and cloned into an expression vector. Peripheral blood mononuclear cells (PBMCs) were isolated from mouse blood and transfected with a recombinant vector that contained the HCG gene by electroporation. In the in vitro experiment: transfected PBMCs were co-cultured with endometrial cells and assessed for blastocyst attachment, then the expression gene was investigated by real-time polymerase chain reaction. In the in vivo experiment: transfected PBMCs were transmitted into uterine horns in the pregnant mice, on day 1.5 post-coitum to assess the number of implantation sites. IVF, in vitro fertilization.

Figure 2.
(A) Colony polymerase chain reaction for the confirmation of HCG gene cloning in the plasmid expressing green fluorescent protein-N1 (PEGFP-N1) vector. Lane 1: DNA ladder (1 kb). Lane 2: positive clone (670 bp). (B) Enzymatic digestion of the PEGFP-N1-HCG vector (recombinant vector) by BamHI and EcoRI. Lane 1: DNA ladder (500 bp) and Lane 2: the lower band (560 bp) is the HCG gene, and the upper band (4,700 bp) is the PEGFP-N1 vector. (C) The enzyme-linked immunosorbent assay method was applied to determine the concentration of produced HCG in the different groups. HCG, human chorionic gonadotropin; PBMC-pE, transfected PBMC with empty pEGFP-N1 vector; PBMC-pE-HCG, transfected PBMC with pEGFP-N1-HCG vector.

Figure 3.
Assessment of blastocyst attachment in the co-cultured endometrial cells and peripheral blood mononuclear cells (PBMCs). (A-C) Attachment and migration of the blastocysts occurred within 24–72 hours. (D) The percentage of blastocyst attachment in the study groups. Values are presented as mean±standard deviation. a)P=0.014 compared with control group; b)P=0.002 compared with control group; analysis of variance, Tukey test. Endo, endometrial cells; HCG, human chorionic gonadotropin.
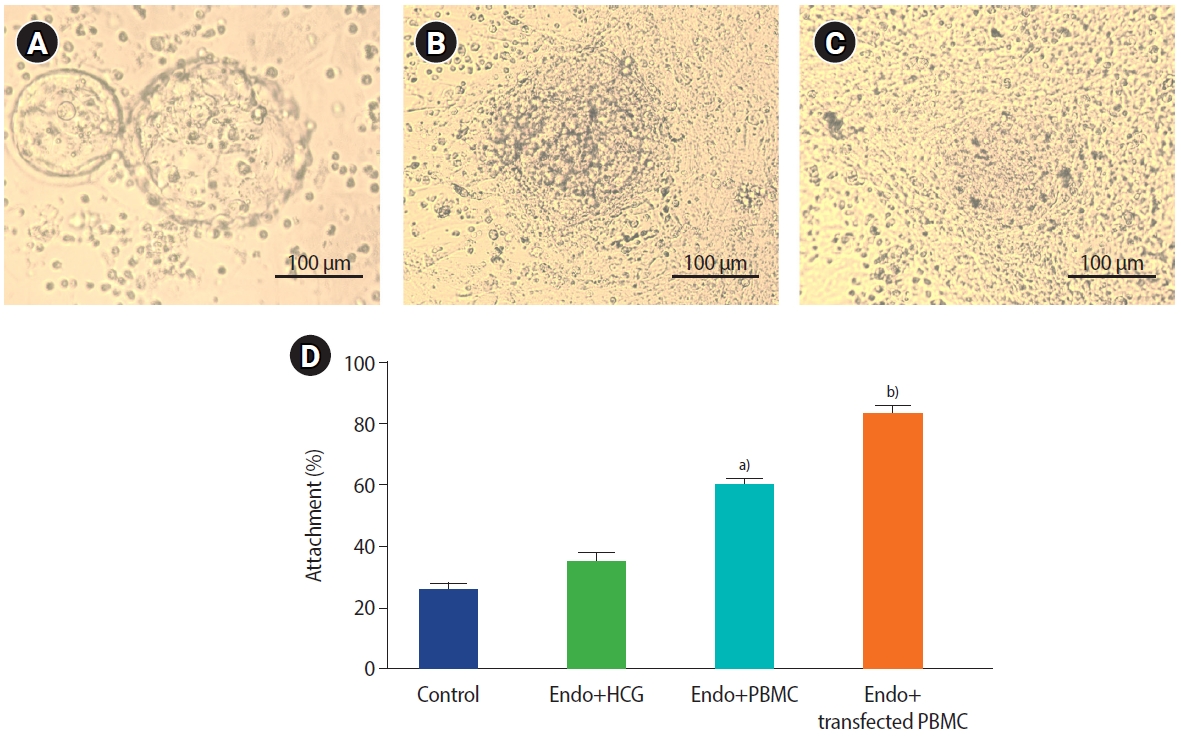
Figure 4.
In vivo animal experiments. The histogram shows pregnancy rates. (A) Implantation sites on day 7.5 post-coitum in the different groups. In the transfected peripheral blood mononuclear cells (PBMCs) with recombinant vector (human chorionic gonadotropin [HCG]-producing PBMCs), the pregnancy rate and the number of implantation sites were significantly higher than in the other groups (analysis of variance, Tukey test). The number of pregnant mice out of the tested mice is shown, for example (8/9) means that out of 9 mice tested, 8 mice became pregnant. Values are presented as mean±standard deviation. (B-F) Examples of implantation sites in the uteri of studied pregnant mice in each group on day 7.5 post-coitum: (B) control, (C) embryo implantation dysfunction (EID), (D) EID+PBMCs, (E) EID+HCG, (F) EID+PBMC+HCG (HCG-producing PBMCs) groups. Control group, 16 implanted embryos; EID group, 3 implanted embryos; EID+HCG group, 6 implanted embryos; EID+PBMC group, 9 implanted embryos and EID with HCG-producing PBMC group, 12 implanted embryos.
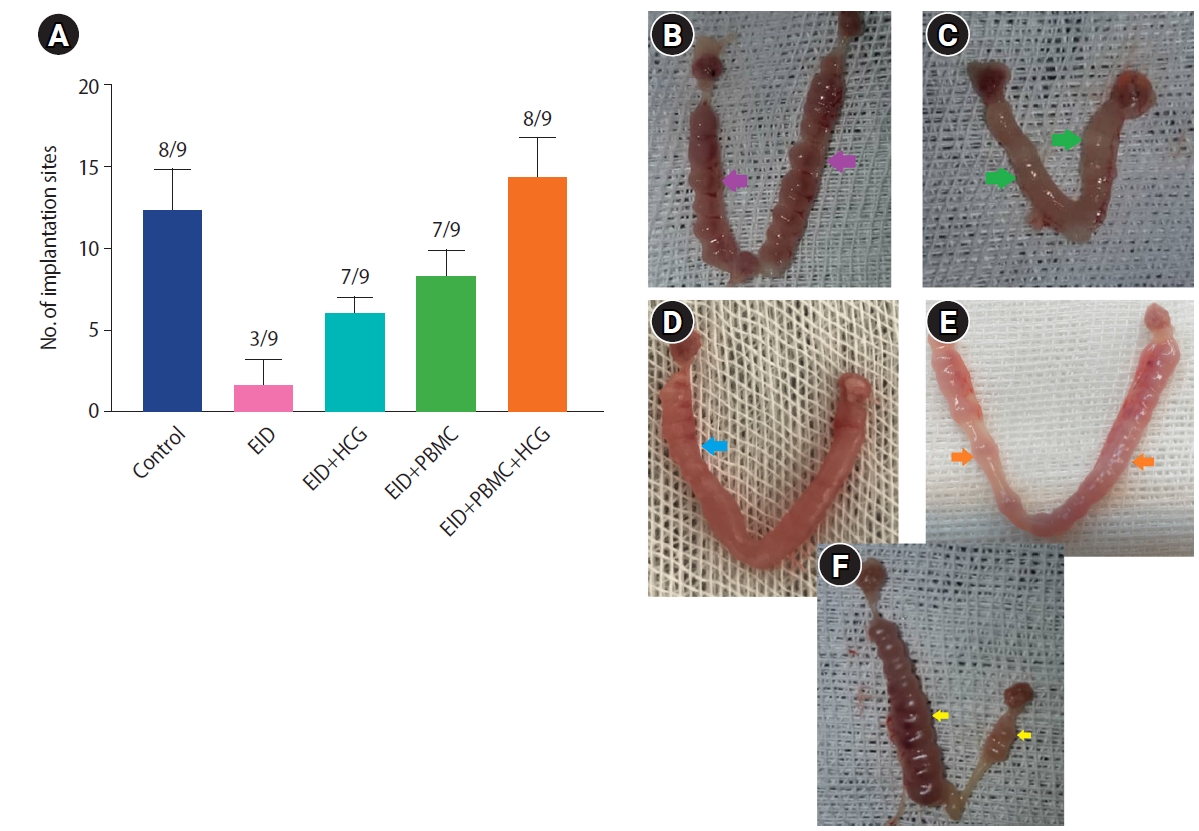
Figure 5.
Relative expression levels of (A) interleukin 1β (IL-1β), (B) interleukin 6 (IL-6), (C) leukemia inhibitory factor (Lif), (D) vascular endothelial growth factor (Vegf), (E) Janus kinase 2 (Jak2), (F) signal transducer and activator of transcription 3 (Stat3), and (G) matrix metalloproteinase 9 (Mmp-9), mRNA expression in the different groups. The mRNA levels of genes were analyzed with qPCR (the reference gene was beta-2 microglobulin). The histogram demonstrates that the expression of genes in the human chorionic gonadotropin (HCG)-producing peripheral blood mononuclear cells (PBMCs) group was significantly higher than in the other groups. Values are presented as mean±standard deviation. EID, embryo implantation dysfunction; EID+recombinant vector, EID with HCG-producing PBMC; qPCR, quantitative real-time polymerase chain reaction. a)p≤0.02; b)p≤0.003; paired t-test.

Table 1.
List of primers employed in quantitative real-time polymerase chain reaction
Table 2.
Developmental competence rate for mouse embryos after IVF
| Group | Number of oocytes | Two‐cell stage (%) | Four‐cell stage (%) | Eight‐cell stage (%) | Morula (%) | Blastocyst (%) | p-valuea) |
|---|---|---|---|---|---|---|---|
| IVF | 120 | 95.8±0.61 | 91.6±1.8 | 90.0±2.5 | 87.5±2.65 | 84.1±1.7 | 0.044 |
References
1. Yakin K, Oktem O, Urman B. Intrauterine administration of peripheral mononuclear cells in recurrent implantation failure: a systematic review and meta-analysis. Sci Rep 2019;9:3897.




2. Bashiri A, Halper KI, Orvieto R. Recurrent implantation failure: update overview on etiology, diagnosis, treatment and future directions. Reprod Biol Endocrinol 2018;16:121.




4. Noyola-Martínez N, Halhali A, Barrera D. Steroid hormones and pregnancy. Gynecol Endocrinol 2019;35:376-84.


5. Yu N, Zhang B, Xu M, Wang S, Liu R, Wu J, et al. Intrauterine administration of autologous peripheral blood mononuclear cells (PBMCs) activated by HCG improves the implantation and pregnancy rates in patients with repeated implantation failure: a prospective randomized study. Am J Reprod Immunol 2016;76:212-6.



6. Morelli SS, Mandal M, Goldsmith LT, Kashani BN, Ponzio NM. The maternal immune system during pregnancy and its influence on fetal development. Res Rep Biol 2015;6:171-89.

7. Nwabuobi C, Arlier S, Schatz F, Guzeloglu-Kayisli O, Lockwood CJ, Kayisli UA. hCG: biological functions and clinical applications. Int J Mol Sci 2017;18:2037.



8. Yoshioka S, Fujiwara H, Nakayama T, Kosaka K, Mori T, Fujii S. Intrauterine administration of autologous peripheral blood mononuclear cells promotes implantation rates in patients with repeated failure of IVF-embryo transfer. Hum Reprod 2006;21:3290-4.


9. Pourmoghadam Z, Soltani-Zangbar MS, Sheikhansari G, Azizi R, Eghbal-Fard S, Mohammadi H, et al. Intrauterine administration of autologous hCG- activated peripheral blood mononuclear cells improves pregnancy outcomes in patients with recurrent implantation failure; a double-blind, randomized control trial study. J Reprod Immunol 2020;142:103182.


10. Okitsu O, Kiyokawa M, Oda T, Miyake K, Sato Y, Fujiwara H. Intrauterine administration of autologous peripheral blood mononuclear cells increases clinical pregnancy rates in frozen/thawed embryo transfer cycles of patients with repeated implantation failure. J Reprod Immunol 2011;92:82-7.


11. Li S, Wang J, Cheng Y, Zhou D, Yin T, Xu W, et al. Intrauterine administration of hCG-activated autologous human peripheral blood mononuclear cells (PBMC) promotes live birth rates in frozen/thawed embryo transfer cycles of patients with repeated implantation failure. J Reprod Immunol 2017;119:15-22.


12. Madkour A, Bouamoud N, Louanjli N, Kaarouch I, Copin H, Benkhalifa M, et al. Intrauterine insemination of cultured peripheral blood mononuclear cells prior to embryo transfer improves clinical outcome for patients with repeated implantation failures. Zygote 2016;24:58-69.


13. Rezaee D, Bandehpour M, Kazemi B, Salehi M. Role of intrauterine administration of transfected peripheral blood mononuclear cells by GM-CSF on embryo implantation and pregnancy rate in mice. Mol Hum Reprod 2020;26:101-10.



14. Rezaee D, Hosseini S, Jajarmi V, Salehi M. Efficient of toll‐like receptor 4 knockout in mouse zygotes by CRISPER/Cas9. Nov Biomed 2021;9:132-7.
15. Yu N, Yang J, Guo Y, Fang J, Yin T, Luo J, et al. Intrauterine administration of peripheral blood mononuclear cells (PBMCs) improves endometrial receptivity in mice with embryonic implantation dysfunction. Am J Reprod Immunol 2014;71:24-33.


16. Dehghan Z, Mohammadi-Yeganeh S, Rezaee D, Salehi M. MicroRNA-21 is involved in oocyte maturation, blastocyst formation, and pre-implantation embryo development. Dev Biol 2021;480:69-77.


17. Teles A, Zenclussen AC. How cells of the immune system prepare the endometrium for implantation. Semin Reprod Med 2014;32:358-64.


18. Ledee N, Petitbarat M, Chevrier L, Vitoux D, Vezmar K, Rahmati M, et al. The uterine immune profile may help women with repeated unexplained embryo implantation failure after in vitro fertilization. Am J Reprod Immunol 2016;75:388-401.




19. Hajipour H, Nejabati HR, Latifi Z, Hamdi K, Bahrami-Asl Z, Fattahi A, et al. Lymphocytes immunotherapy for preserving pregnancy: mechanisms and Challenges. Am J Reprod Immunol 2018;80:e12853.



20. Ahmadi M, Abdolmohammadi-Vahid S, Ghaebi M, Aghebati-Maleki L, Dolati S, Farzadi L, et al. Regulatory T cells improve pregnancy rate in RIF patients after additional IVIG treatment. Syst Biol Reprod Med 2017;63:350-9.


21. Strakova Z, Mavrogianis P, Meng X, Hastings JM, Jackson KS, Cameo P, et al. In vivo infusion of interleukin-1beta and chorionic gonadotropin induces endometrial changes that mimic early pregnancy events in the baboon. Endocrinology 2005;146:4097-104.

22. Staun-Ram E, Shalev E. Human trophoblast function during the implantation process. Reprod Biol Endocrinol 2005;3:56.




23. Zhu JY, Pang ZJ, Yu YH. Regulation of trophoblast invasion: the role of matrix metalloproteinases. Rev Obstet Gynecol 2012;5:e137-43.


24. Rosario GX, Stewart CL. The multifaceted actions of leukaemia inhibitory factor in mediating uterine receptivity and embryo implantation. Am J Reprod Immunol 2016;75:246-55.


25. Salleh N, Giribabu N. Leukemia inhibitory factor: roles in embryo implantation and in nonhormonal contraception. ScientificWorldJournal 2014;2014:201514.




26. Camargo-Diaz F, Garcia V, Ocampo-Barcenas A, Gonzalez-Marquez H, Lopez-Bayghen E. Colony stimulating factor-1 and leukemia inhibitor factor expression from current-cycle cannula isolated endometrial cells are associated with increased endometrial receptivity and pregnancy. BMC Womens Health 2017;17:63.


27. Suman P, Malhotra SS, Gupta SK. LIF-STAT signaling and trophoblast biology. JAKSTAT 2013;2:e25155.



28. Guo X, Yi H, Li TC, Wang Y, Wang H, Chen X. Role of vascular endothelial growth factor (VEGF) in human embryo implantation: clinical implications. Biomolecules 2021;11:253.



29. Rockwell LC, Pillai S, Olson CE, Koos RD. Inhibition of vascular endothelial growth factor/vascular permeability factor action blocks estrogen-induced uterine edema and implantation in rodents. Biol Reprod 2002;67:1804-10.

30. Fujiwara H. Do circulating blood cells contribute to maternal tissue remodeling and embryo-maternal cross-talk around the implantation period? Mol Hum Reprod 2009;15:335-43.


- TOOLS







