 |
 |
- Search
| Clin Exp Reprod Med > Volume 49(3); 2022 > Article |
|
Abstract
Objective
The aim of this study was to assess the correlation of oocyte number with serum anti-Müllerian hormone (AMH) levels measured by two automated methods (Access or Elecsys) in fresh stimulated in vitro fertilization (IVF) cycles.
Methods
In this retrospective study at a university hospital, data were collected from 243 fresh stimulated IVF cycles performed from August 2016 to December 2020. The serum AMH level was measured by Access in 120 cycles and by Elecsys in 123 cycles. The cut-off of serum AMH for prediction of poor responders (three or fewer oocytes) or high responders (15 or more oocytes) was calculated by the receiver operating characteristic curve analysis.
Results
For the two automated methods, the following equations were derived: total oocyte number=2.378+1.418×(Access-AMH) (r=0.645, p<0.001) and total oocyte number=2.417+2.163×(Elecsys-AMH) (r=0.686, p<0.001). The following combined equation could be derived: (Access-AMH)=0.028+1.525×(Elecsys-AMH). To predict poor responders, the cut-off of Access-AMH was 1.215 ng/mL (area under the curve [AUC], 0.807; 95% confidence interval [CI], 0.730–0.884; p<0.001), and the cut-off of Elecsys-AMH was 1.095 ng/mL (AUC, 0.848; 95% CI, 0.773–0.923; p<0.001). To predict high responders, the cut-off of Access-AMH was 3.450 ng/mL (AUC, 0.922; 95% CI, 0.862–0.981; p<0.001), and the cut-off of Elecsys-AMH was 2.500 ng/mL (AUC, 0.884; 95% CI, 0.778–0.991; p<0.001).
Anti-Müllerian hormone (AMH) is a dimeric glycoprotein that is a member of the transforming growth factor β family. It is produced in the Sertoli cells of testes and plays a role in male sexual differentiation [1]. AMH is also produced in the granulosa cells of pre-antral and small antral follicles in women [2,3]. The serum AMH level is widely used to assess ovarian reserve and predict the ovarian response to an exogenous gonadotropin in in vitro fertilization (IVF) cycles [1,4-9]. It has been shown that the serum AMH level has similar or better performance than the antral follicle count (AFC) for predicting the oocyte yield in stimulated IVF cycles [10].
Measurement of serum AMH by enzyme-linked immunosorbent assays was first reported in the 1990s; this so-called first-generation AMH assay was developed and produced both by Diagnostic Systems Lab (DSL) and Immunotech (IOT). Each company’s assay used different primary antibodies against AMH and different calibrators, resulting in different values when the same sample was analyzed [11]. The DSL antibody and IOT standard calibrators were later combined in 2010, and the second-generation (Gen II, original) assay was developed by Immunotech Beckman Coulter [12,13]. Shortly thereafter, the revised Gen II assay was introduced by adding a pre-mix step with new AMH reference ranges in 2013 [3]. The revised Gen II-AMH level was usually somewhat higher than the original Gen II-AMH level [3].
In 2015, fully automated AMH assays were released by Beckman Coulter (Access) and by Roche (Elecsys) [14,15]. The automated assay uses recombinant AMH as a calibrator, thereby reducing the test time and improving sample instability or variability. Ultimately, the reproducibility was quite substantially improved compared to the previous manual methods [16-19]. Tadros et al. [19] reported that, on average, Access-AMH levels were 16% lower and Elecsys-AMH levels were 20% lower than the levels reported using the revised Gen II assay in patients with reduced AFC. Therefore, the AMH levels measured by the automated assays are considered to be similar to those obtained using the original Gen II assay [18]. However, Access-AMH showed a better correlation with oocyte number than the revised Gen II assay [15,18]. Theoretically, both Access-AMH and Elecsys-AMH levels in a single person would be expected to be similar because both methods use the same antibody. Nonetheless, the possibility of a difference in these measured values in a single person still exists, since Access-AMH uses five approximate calibration points (0.16, 0.6, 4, 10, and 24 ng/mL), but Elecsys-AMH uses three points.
In previous studies, the Access-AMH levels and Elecsys-AMH levels in the same patient showed a significant correlation, and there was a tendency for higher levels to be measured using Access-AMH than using Elecsys-AMH [14,20,21]. In the present study, we evaluated the association of oocyte number with Access-AMH levels or Elecsys-AMH levels in different cohorts of patients undergoing stimulated IVF cycles, and determined the cut-off of Access-AMH or Elecsys-AMH to predict poor responders (3 or fewer oocytes) or high responders (15 or more oocytes).
We selected 243 fresh IVF cycles performed between August 2016 and December 2020 at Seoul National University Bundang Hospital. The initial indication of IVF was unexplained infertility in 48 couples, diminished ovarian reserve in 46 couples, tubal factor infertility in 38 couples, endometriosis in 24 couples, male factor infertility in 22 couples, and mixed-cause infertility in 65 couples. The Institutional Review Board of the Seoul National University Bundang Hospital approved the use of patients’ medical records and IVF laboratory data (IRB No. B-2110-714-101). As this study was a retrospective study, only the data on the procedure already performed were used, so patient consent was omitted.
In all cycles, full stimulation with recombinant follicle-stimulating hormone (FSH) with or without purified human menopausal gonadotropin (hMG) (excluding mild stimulation or natural cycle) was used, and the serum AMH level was measured within 1 year before ovarian stimulation by Access (Beckman Coulter, Brea, CA, USA) in 120 cycles and by Elecsys (Roche Diagnostics, Basel, Switzerland) in 123 cycles. The AMH measurement method was assigned at random or at the physician's preference.
The Access-AMH and Elecsys-AMH assays are automated immunoassays that utilize chemiluminescence for detection. They are not susceptible to interference by serum complement [22]. The total duration of assay is 39 minutes for Access-AMH and 18 minutes for Elecsys-AMH. The measurement range of Access-AMH is 0.02–24.00 ng/mL, and the intra- and inter-assay coefficients of variation are ≤1.7% and ≤2.8% according to the manufacturer’s instructions. For Elecsys-AMH, the measurement range is 0.01–23.00 ng/mL, and the intra- and inter-assay coefficients of variation are ≤2.6% and ≤3.9%, respectively [22].
Ovarian stimulation was performed with recombinant FSH (Gonal-f; Merck Serono, Darmstadt, Germany) (142 cycles), recombinant FSH and purified hMG (Menopur; Ferring Pharmaceuticals, Kiel, Germany) (7 cycles), or recombinant FSH and recombinant luteinizing hormone (Pergoveris, Merck Serono) (17 cycles). A flexible gonadotropin-releasing hormone (GnRH) antagonist was used for pituitary suppression in all IVF cycles. Briefly, gonadotropins (according to the serum AMH level and individual ovarian response of previous cycles) were started on menstrual day 2–4 and the doses were adjusted. When the leading follicle reached a diameter of 14 mm, cetrorelix (Cetrotide, 0.25 mg/day; Merck Serono) was started and when the leading follicle reached a diameter of 18–19 mm, 250 μg or 500 μg of recombinant human chorionic gonadotropin (hCG; Ovidrel, Merck-Serono) (171 cycles), 5,000 IU of urinary hCG (IVF-C; LG Chemical, Seoul, Korea) (2 cycles), a GnRH agonist (Decapeptyl [0.2 mg], Ferring) (3 cycles), or 250 μg of recombinant hCG with a GnRH agonist (Decapeptyl [0.2 mg]) (67 cycles) was administered for final triggering. Oocytes were retrieved 35–36 hours later. The total oocyte number and the mature oocyte number were recorded. In most cases, oocyte maturity could be easily evaluated under stereomicroscopy on the basis of the cumulus pattern. In situations where the maturity was unclear due to dark cumulus cells or blood clots, the oocytes were denuded using 85 IU/mL hyaluronidase (Cook, Bloomington, IN, USA) and mechanical pipetting. Mature oocytes were defined according to the presence of the first polar body and absence of a germinal vesicle.
Statistical analysis was performed using IBM SPSS ver. 25.0 (IBM Corp., Armonk, NY, USA). All variables were presented as mean±standard deviation. Correlations between pairs of numeric parameters (such as serum AMH level, serum estradiol level at triggering day, the number of total or mature oocytes, and ovarian sensitivity [OS]) were assessed by the Spearman rank test. The OS was calculated in two ways; The OS-TO was defined as the total oocyte number per 500 IU of total gonadotropins, and the OS-MO was defined as the mature oocyte number per 500 IU of total gonadotropins. Equations were derived for the relationships between pairs of numeric parameters through linear regression analysis. The cut-off of serum AMH for the prediction of poor responders (3 or fewer oocytes) or high responders (15 or more oocytes) was calculated using receiver operating characteristic curve analysis. A p-value <0.05 was considered to indicate statistical significance.
The basal characteristics of two cohorts are shown in Table 1. The mean total gonadotropin dose was significantly higher in the Elecsys-AMH cohort than in the Access-AMH cohort. The correlation coefficients between serum AMH levels and five stimulation outcomes are presented in Table 2. Serum estradiol level at triggering day, the number of total or mature oocytes, OS-TO, and OS-MO were all positively associated with the Access-AMH level or Elecsys-AMH level, with statistical significance.
Figure 1 shows the linear regression lines between the Access-AMH level or Elecsys-AMH level and the total oocyte number. Linear regression analysis derived four equations to show the relationships between four stimulation outcomes and AMH levels (Table 3). For each stimulation outcome, when two equations (from Access-AMH and Elecsys-AMH) were combined, a total of four equations to show the correlations between the Access-AMH level and the Elecsys-AMH level could be derived. In the calculated four equations, the Access-AMH level was usually higher than the Elecsys-AMH level. For example, using the equations for the total oocyte number, the Access-AMH level was 1.553 ng/mL when the Elecsys-AMH level was 1.0 ng/mL. When using the equation for the mature oocyte number, an Access-AMH level of 1.269 ng/mL corresponded to an Elecsys-AMH level of 1.0 ng/mL.
For the prediction of poor responders, the cut-off of Access-AMH was 1.215 ng/mL (area under the curve [AUC], 0.807; 95% confidence interval [CI], 0.730–0.884; p<0.001), and the cut-off of Elecsys-AMH was 1.095 ng/mL (AUC, 0.848; 95% CI, 0.773–0.923; p<0.001) (Table 4, Figure 2). For the prediction of high responders, the cut–off of Access-AMH was 3.450 ng/mL (AUC, 0.922; 95% CI, 0.862–0.981; p<0.001), and the cut-off of Elecsys-AMH was 2.500 ng/mL (AUC, 0.884; 95% CI, 0.778–0.991; p<0.001) (Table 5, Figure 3).
In the present study, we demonstrated that two fully automated AMH measurements could well predict the oocyte number in infertile women who underwent stimulated IVF cycles. No previous study has investigated whether there is a difference in serum AMH levels between these two methods for fully automated measurements of AMH. Although the data are from different cohorts, a correlation between Access-AMH and Elecsys-AMH was identified, assuming the same number of oocytes. We believe that our results will be very useful in interpreting AMH levels measured by other methods at centers that usually use only one method.
The Access-AMH level was usually higher than the Elecsys-AMH level. However, considering the OS-E and OS-MO, it is thought that interoperability would be difficult because the relationship between the two was not consistent at low AMH values.
In addition, the trend for Access-AMH levels to be higher than Elecsys-AMH levels could be related to the higher total gonadotropin dose in the Elecsys-AMH group than in the Access-AMH group. According to European Society of Human Reproduction and Embryology (ESHRE) guidelines on ovarian stimulation for IVF and intracytoplasmic sperm injection, AMH and AFC could predict the ovarian response well during ovarian stimulation. They recommended establishing the FSH starting dose considering AMH and AFC [10]. Due to the retrospective nature of this study and differences in physicians’ preferences across groups, it is not possible to draw a firm conclusion that the total gonadotropin dose fully explains the difference in AMH; therefore, additional research is needed to address this question in the future.
La Marca et al. [20] reported that the Access-AMH level was usually higher than the Elecsys-AMH level. When calculating the dose of follitropin alfa, Access-AMH value was used instead of the Elecsys-AMH value, and a ≥15% difference in the starting dose occurred in only 2 of 113 patients. When calculating the dose of follitropin delta, the Access-AMH value was used instead of the Elecsys-AMH value, and a ≥15% difference in the starting dose occurred in 21 of 113 patients. In general, when using follitropin delta, the Elecsys-AMH level is considered the gold standard. Considering the results of this paper, the choice between using Elecsys-AMH or Access-AMH values appears to have little effect on the determination of the correct FSH dose used for ovarian stimulation. The authors suggest that the two most widely used automated AMH assays, Elecsys and Access, have modest differences in values, and the clinical significance of this study’s results lies in the reliability of the interchangeable use of AMH values obtained from both assays.
In the present study, the correlation coefficient of Access-AMH with the total oocyte number was 0.645, and that of Elecsys-AMH with the total oocyte number was 0.686. Asada et al. [15] reported that the correlation coefficient of Access-AMH with the total oocyte number was 0.655, which is very similar to our result. Homburg et al. [22] reported a correlation coefficient of 0.48 between Access-AMH and the total oocyte number.
Our study also showed that both automated methods for serum AMH measurement had good performance in predicting poor and high responders in fresh stimulated IVF cycles. Based on our observations, the cut-off of Access-AMH was 1.215 ng/mL and the cut-off of Elecsys-AMH was 1.095 ng/mL for predicting poor responders. The cut-off of Access-AMH was 3.450 ng/mL and the cut-off of Elecsys-AMH was 2.500 ng/mL for predicting high responders.
In the Bologna criteria defining poor responders, a serum AMH level <0.5–1.1 ng/mL was presented as one of the criteria [23]. Broer et al. [24] also presented the cut-off of AMH for predicting poor or high responders in their meta-analysis as 2.0 ng/mL (95% CI, 0.1–5.7 ng/mL) and 4.8 ng/mL (95% CI, 1.3–10.2 ng/mL; p<0.001), respectively. In their study, AMH was all measured by IOT. We also previously reported that the cut-offs of AMH levels measured using IOT were 1.08 ng/mL and 3.57 ng/mL, respectively, for predicting poor and high responders (≥20 oocytes) [25].
After the introduction of automated methods, the cut-off of Access-AMH or Elecsys-AMH for prediction of poor or high responders should be reset. Bosch et al. [10] suggested that the FSH dose and the drug and dose for triggering should be different for ovarian stimulation in poor and high responders, considering the AMH level and AFC. However, they also reported that there was no consistent definition of poor and high responders.
Baker et al. [26] studied whether Access-AMH could be used to predict poor ovarian responders. The mean value of Access-AMH among patients with poor ovarian response to ovarian stimulation, defined as 4 or fewer oocytes retrieved, was 0.74 ng/mL, whereas the cut-off was 3.20 ng/mL for normal to high responders. The cut-off for predicting poor ovarian response at 90% specificity was 0.93 ng/mL (sensitivity, 74.1%; specificity, 90%). There was no AMH cut-off value for high responders, and the AMH cut-off value for an AFC >15 was 1.75 ng/mL (sensitivity, 90%; specificity, 59.1%). Homburg et al. [22] reported that the cut-offs of Access-AMH were 0.77 ng/mL for poor responders and 2.184 ng/mL for high responders (>15 oocytes). In that report, the serum AMH levels of 1,787 and 1,258 patients at two different sites were measured by the Access-AMH method. The cut-off level of Access-AMH for predicting poor ovarian response and high ovarian response in our study was somewhat higher than that of Baker et al. [26] and Homburg et al. [22]. This may have been because the average age of the Access group in our study was 39.94 years, which is higher than in previous studies.
Iliodromiti et al. [27] systematically searched and analyzed the literature measured by two automated measures in the same patient cohort. They found that Access-AMH values were higher than those obtained using Elecsys, and the correlation was linear (Access=−0.05+1.10×Elecsys). Access-AMH showed a higher value on average by about 10% compared to Elecsys-AMH, and when using the AMH value measured by Access-AMH, it was reported that attention should be paid because the patients would receive a lower dose of follitropin delta based on the Access-AMH levels.
Tan et al. [28] prospectively measured and analyzed both Access-AMH and Elecsys-AMH in 43 infertile women aged 21 to 45 years. They reported that the cut-off of AMH for predicting poor ovarian response was 2.23 ng/mL for Access-AMH and 2.02 ng/mL for Elecsys-AMH. Furthermore, the cut-off of AMH for predicting high ovarian response was reported to be 5.19 ng/mL for Access-AMH and 4.60 ng/mL for Elecsys-AMH. The cut-off values in Tan’s study were all higher than in our study, which is probably due to the small sample size. However, the results are consistent with previous papers, which reported that Access-AMH showed slightly higher values than Elecsys-AMH.
It has been reported that very low values of AMH (1.5 pmol/L, which is equivalent to 0.21 ng/mL [15]) for Elecsys-AMH can predict cycle cancellation, and the cut-off value of Elecsys-AMH for a low oocyte yield (defined as ≤3 oocytes) was 0.56 ng/mL [29]. That result also confirmed that the cut-off value of Elecsys-AMH was somewhat higher than that of our study. It is presumed that these results were caused by differences in patient groups and stimulation protocols.
The limitations of our study are related to its retrospective nature. The AMH measurement method was determined by the physician's preference, and the interval between AMH measurement and oocyte pick-up was wide, although within 1 year. However, as in previous studies, Access-AMH showed slightly higher values than Elecsys-AMH. The finding that both AMH measurement methods predicted poor and high ovarian response is also consistent with previous studies.
In conclusion, although there is a slight difference between the two methods, both automated AMH measurement methods show good correlations with the number of retrieved oocytes and predict poor and high ovarian response relatively well. In the future, a large-scale prospective study is needed to clarify the differences between the two test methods.
Notes
Conflict of interest
Byung Chul Jee is an Editor-in-Chief and Seul Ki Kim is an Associate Editor of the journal, but they were not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Figure 1.
Linear regression lines to show correlations between the total oocyte number and serum anti-Müllerian hormone (AMH) levels measured by Access (blue color) and Elecsys (red color).
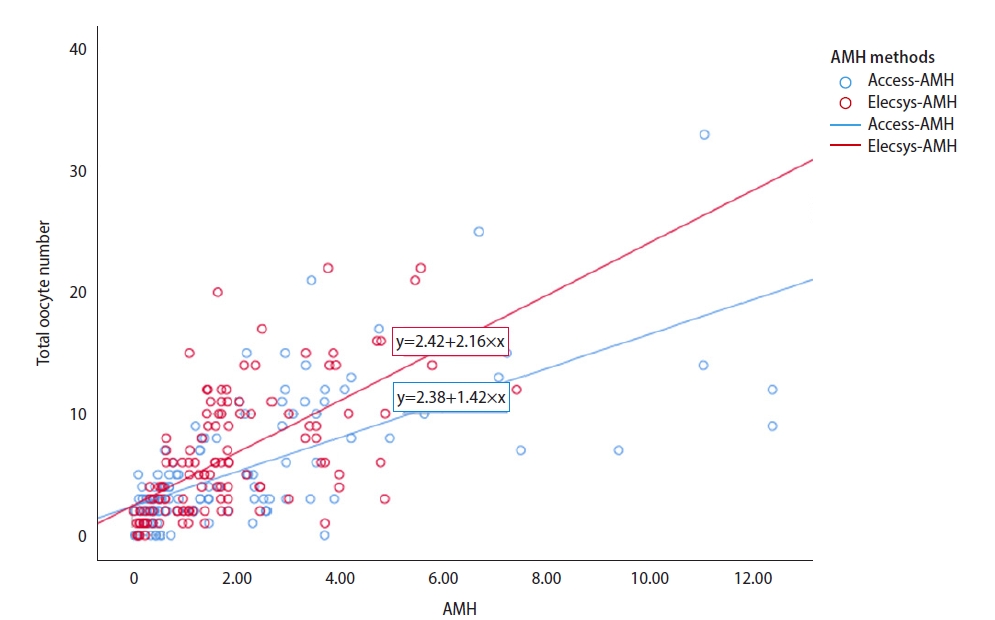
Figure 2.
Receiver operating characteristic (ROC) curves of serum anti-Müllerian hormone (AMH) levels for the prediction of poor ovarian response (total oocytes ≤3).
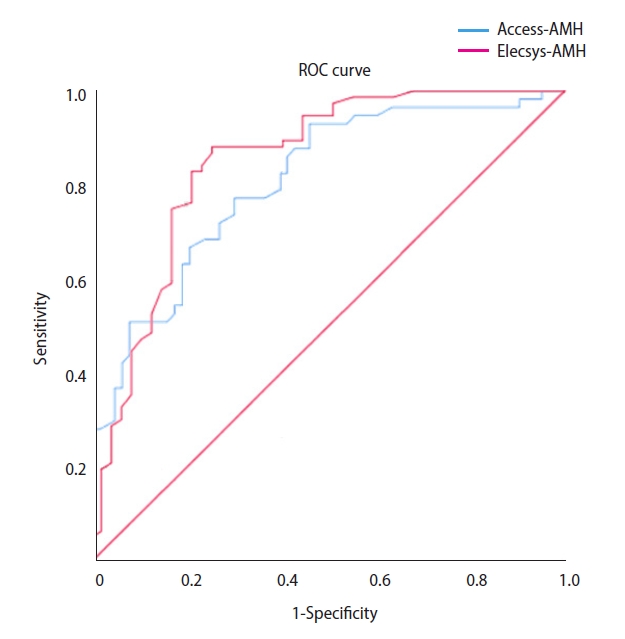
Figure 3.
Receiver operating characteristic (ROC) curves of serum anti-Müllerian hormone (AMH) levels for the prediction of high ovarian response (total oocytes ≥15).
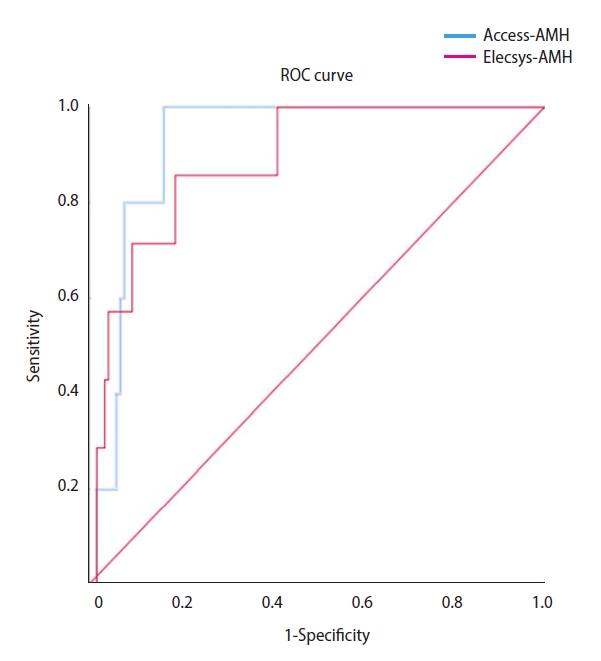
Table 1.
Basal clinical characteristics of Access-AMH cohort and Elecsys-AMH cohort and their stimulation outcomesa)
| Variable | Access-AMH cohort (120 cycles) | Elecsys-AMH cohort (123 cycles) | p-value |
|---|---|---|---|
| Female age (yr) | 36.8±4.9 | 37.1±4.5 | 0.620 |
| Male age (yr) | 39.9±5.4 | 39.1±5.1 | 0.689 |
| Cause of infertility | <0.001 | ||
| Male factor | 12 (10.0) | 10 (8.1) | |
| Female factor | 94 (78.3) | 70 (56.9) | |
| Combined | 5 (4.2) | 4 (3.3) | |
| Unexplained | 9 (7.5) | 39 (31.7) | |
| Serum AMH level (ng/mL) | 2.19±2.54 | 1.82±1.48 | 0.253 |
| Duration between measurement of serum AMH and oocyte pick-up (day) | 87.2±93.9 | 100.1±96.7 | 0.466 |
| Total dose of gonadotropin (IU) | 2,279±658 | 2,535±632 | 0.004 |
| Serum E level at triggering day | 1,214±1,036 | 1,481±1,077 | 0.079 |
| No. of total oocyte (TO) | 5.5±5.4 | 6.4±5.2 | 0.146 |
| No. of mature oocyte (MO) | 3.3±3.4 | 3.8±3.5 | 0.140 |
| Ovarian sensitivity-TOb) | 1.49±1.97 | 1.42±1.38 | 0.877 |
| Ovarian sensitivity-MOc) | 0.88±1.13 | 0.84±0.89 | 0.948 |
Table 2.
Correlations between serum AMH level and five stimulation outcomesa)
Table 3.
The equations between serum AMH level and four stimulation outcomes and derivation of four equations between two serum AMH levels
Table 4.
Results of receiver operating characteristic curves of serum AMH level for prediction of poor ovarian response (total oocytes ≤3)
Table 5.
Results of receiver operating characteristic curves of serum AMH level for prediction of high ovarian response (total oocytes ≥15)
References
1. Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update 2014;20:370-85.

2. Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod 2004;10:77-83.

3. Bonifacio M, Bradley CK, Karia S, Livingstone M, Bowman MC, McArthur SJ. The original Beckman Coulter Generation II assay significantly underestimates AMH levels compared with the revised protocol. J Assist Reprod Genet 2015;32:1691-6.




4. Kevenaar ME, Meerasahib MF, Kramer P, van de Lang-Born BM, de Jong FH, Groome NP, et al. Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology 2006;147:3228-34.


5. La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update 2010;16:113-30.


6. Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril 2011;95:170-5.


7. Nelson SM, Fleming R, Gaudoin M, Choi B, Santo-Domingo K, Yao M. Antimüllerian hormone levels and antral follicle count as prognostic indicators in a personalized prediction model of live birth. Fertil Steril 2015;104:325-32.


8. Magnusson A, Olerod G, Thurin-Kjellberg A, Bergh C. The correlation between AMH assays differs depending on actual AMH levels. Hum Reprod Open 2017;2017:hox026.



9. Pacheco A, Cruz M, Iglesias C, Garcia-Velasco JA. Very low anti-Müllerian hormone concentrations are not an independent predictor of embryo quality and pregnancy rate. Reprod Biomed Online 2018;37:113-9.


10. Ovarian Stimulation TEGGO, Bosch E, Broer S, Griesinger G, Grynberg M, Humaidan P, et al. ESHRE guideline: ovarian stimulation for VF/ICSI. Hum Reprod Open 2020;2020:hoaa009.


11. Nelson SM, La Marca A. The journey from the old to the new AMH assay: how to avoid getting lost in the values. Reprod Biomed Online 2011;23:411-20.


12. Wallace AM, Faye SA, Fleming R, Nelson SM. A multicentre evaluation of the new Beckman Coulter anti-Mullerian hormone immunoassay (AMH Gen II). Ann Clin Biochem 2011;48(Pt 4):370-3.
13. Craciunas L, Roberts SA, Yates AP, Smith A, Fitzgerald C, Pemberton PW. Modification of the Beckman-Coulter second-generation enzyme-linked immunosorbent assay protocol improves the reliability of serum antimüllerian hormone measurement. Fertil Steril 2015;103:554-59.


14. Nelson SM, Pastuszek E, Kloss G, Malinowska I, Liss J, Lukaszuk A, et al. Two new automated, compared with two enzyme-linked immunosorbent, antimüllerian hormone assays. Fertil Steril 2015;104:1016-21.


15. Asada Y, Tsuiki M, Sonohara M, Fukunaga N, Hattori Y, Inoue D, et al. Performance of anti-Müllerian hormone (AMH) levels measured by Beckman Coulter Access AMH assay to predict oocyte yield following controlled ovarian stimulation for in vitro fertilization. Reprod Med Biol 2019;18:273-7.




16. Gassner D, Jung R. First fully automated immunoassay for anti-Müllerian hormone. Clin Chem Lab Med 2014;52:1143-52.


17. Anckaert E, Oktem M, Thies A, Cohen-Bacrie M, Daan NM, Schiettecatte J, et al. Multicenter analytical performance evaluation of a fully automated anti-Müllerian hormone assay and reference interval determination. Clin Biochem 2016;49:260-7.


18. Pearson K, Long M, Prasad J, Wu YY, Bonifacio M. Assessment of the Access AMH assay as an automated, high-performance replacement for the AMH Generation II manual ELISA. Reprod Biol Endocrinol 2016;14:8.



19. Tadros T, Tarasconi B, Nassar J, Benhaim JL, Taieb J, Fanchin R. New automated antimüllerian hormone assays are more reliable than the manual assay in patients with reduced antral follicle count. Fertil Steril 2016;106:1800-6.


20. La Marca A, Tolani AD, Capuzzo M. The interchangeability of two assays for the measurement of anti-Müllerian hormone when personalizing the dose of FSH in in-vitro fertilization cycles. Gynecol Endocrinol 2021;37:372-6.


21. Li H, Robertson DM, Burns C, Ledger WL. Challenges in measuring AMH in the clinical setting. Front Endocrinol (Lausanne) 2021;12:691432.



22. Homburg R, Rao U, Malamas F, Palouki P, Gudi A, Shah A, et al. Automated anti-Mullerian hormone measurement: data review to provide insights and interpretation. Gynecol Endocrinol 2021;37:511-4.


23. Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L, et al. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod 2011;26:1616-24.


24. Broer SL, Dolleman M, van Disseldorp J, Broeze KA, Opmeer BC, Bossuyt PM, et al. Prediction of an excessive response in in vitro fertilization from patient characteristics and ovarian reserve tests and comparison in subgroups: an individual patient data meta-analysis. Fertil Steril 2013;100:420-9.


25. Lee JE, Lee JR, Jee BC, Suh CS, Kim KC, Lee WD, et al. Clinical application of anti-Müllerian hormone as a predictor of controlled ovarian hyperstimulation outcome. Clin Exp Reprod Med 2012;39:176-81.



26. Baker VL, Gracia C, Glassner MJ, Schnell VL, Doody K, Coddington CC, et al. Multicenter evaluation of the Access AMH antimüllerian hormone assay for the prediction of antral follicle count and poor ovarian response to controlled ovarian stimulation. Fertil Steril 2018;110:506-13.


27. Iliodromiti S, Salje B, Dewailly D, Fairburn C, Fanchin R, Fleming R, et al. Non-equivalence of anti-Müllerian hormone automated assays-clinical implications for use as a companion diagnostic for individualised gonadotrophin dosing. Hum Reprod 2017;32:1710-5.



28. Tan EC, Chincholkar P, Yu SL, Lim SL, Renuka R, Yong TT, et al. Comparison of automated anti-Müllerian hormone assays and antral follicle count in predicting ovarian response during ovarian stimulation. Fertil Reprod 2019;1:99-105.

29. Grynnerup AG, Lossl K, Pilsgaard F, Lunding SA, Storgaard M, Bogstad JW, et al. Prediction of the lower serum anti-Müllerian hormone threshold for ovarian stimulation prior to in-vitro fertilization using the Elecsys® AMH assay: a prospective observational study. Reprod Biol Endocrinol 2019;17:11.











