 |
 |
- Search
| Clin Exp Reprod Med > Volume 49(2); 2022 > Article |
|
Abstract
Objective
This study examined whether the addition of triple antioxidants (3A)ŌĆö10 ┬ĄM acetyl-L-carnitine, 10 ┬ĄM N-acetyl-L-cysteine, and 5 ┬ĄM ╬▒-lipoic acidŌĆöin freezing-thawing medium during human sperm cryopreservation using the sucrose vitrification (SuV) and liquid nitrogen vapor (Vapor) techniques could improve post-thaw survival of spermatozoa.
Methods
We analyzed 30 samples from healthy human sperm donors. Each sample was allocated into one of five groups: fresh control, SuV, SuV+3A, Vapor, and Vapor+3A. The sperm motility, morphology, viability, intracellular and extracellular reactive oxygen species (ROS) levels, and sperm DNA fragmentation (SDF) were evaluated.
Results
The cryopreserved spermatozoa had significantly reduced percentages of motility (p<0.05) and viability (p<0.05). Antioxidant supplementation non-significantly improved these parameters (p>0.05). No significant differences were found in sperm morphology between the fresh and frozen-thawed groups (p>0.05). After freezing, the extracellular ROS levels in the frozen-thawed groups were significantly higher (p<0.05) than in the fresh group. However, we did not find any differences in intracellular ROS parameters among these groups (p>0.05). The SDF was higher in the SuV and Vapor groups than in the fresh group, but without statistical significance (p=0.075 and p=0.077, respectively).
Cryopreservation of human spermatozoa has been widely used in assisted reproductive technology (ART) for more than 60 years [1]. Since the introducion of cryopreservation, the development of new protocols aiming to optimize the quality of thawed sperm samples has been of major interest to researchers in the field of andrology. It is well known that sperm vitrification using a high concentration of a permeable cryoprotective agent (CPA) can damage sperm cells, inducing osmotic injury and physiological alterations [2]. Other approaches consider the utilization of protocols that avoid the use of CPA as an alternative way to preserve sperm function and viability. For example sucrose, a non-permeating agent, has been successfully used for human sperm cryopreservation [3,4]; however, studies on the relationship between ROS formation and sperm DNA fragmentation (SDF) are limited.
Reactive oxygen species (ROS) are formed as natural byproducts of cellular aerobic metabolism and function as signal molecules that regulate cell-to-cell communication [5]. Normally, a small amount of ROS molecules can be destroyed by the scavenging system itself. However, at the pathological level, ROS can negatively impact cellular function, resulting in sperm damage [6]. Excessive levels of ROS molecules can not only impair sperm motility, but also induce SDF by endonuclease activity via the apoptotic cascade pathway [7].
In previous studies, antioxidants such as acetyl-L-carnitine (ALC), N-acetyl-L-cysteine (NAC), and ╬▒-lipoic acid (ALA) have been shown to exert protective effects individually on several tissues and might be beneficial in mammalian gametes [8-10]. L-carnitine and ALC are found naturally in epididymal fluid. They play a critical role in sperm metabolism, which directly affects sperm motility and fertilization [8,11]. Moreover, ALC improves the in vitro blastocyst development rate in mouse embryos by preventing oxidative stress-induced DNA damage [12]. NAC, a precursor of glutathione (GSH), is widely used thiol-containing antioxidant and modulator of the intracellular redox state [13]. The addition of GSH to the culture medium increased the percentage of fertilization and enhanced embryo development [9]. GSH has an antioxidant defensive capacity during the sperm freezing-thawing process [14]. Sperm quality may improve as a result of cysteineŌĆÖs effect on GSH levels. ALA is a universal antioxidant that acts as a cofactor for mitochondrial enzyme activity [15]. It helps with ATP generation, converts pyruvate to acetyl-CoA by oxidative decarboxylation, and is involved in the citric acid cycle via mitochondrial alpha-ketoglutarate dehydrogenase activity [16]. ALA supplementation in sperm freezing medium improved sperm motility and acrosome integrity, and protected sperm from freezing-thawing-induced DNA damage [10].
In nature, various antioxidant systems act together in concert to provide protection against oxidative stress and promote repair. Previous studies that concentrated on the use of individual antioxidants did not replicate natural conditions. The supplementation of combinations of antioxidants should exert a synergistic effect and provide better protection against oxidative-induced injuries than single antioxidant supplements [17]. Limited research has been conducted on the effects of antioxidant combinations in sperm freezing-thawing medium. A study by Truong and Gardner [18] supplemented ALC, NAC, and ALA in sperm and oocyte washing medium, as well as in embryo culture medium. They showed that a combination of these three antioxidants provided better protection against ROS than their individual counterparts [19]. However, it is not known whether this combination of antioxidants might affect sperm quality after cryopreservation.
The rationale for combining the three antioxidants is as follows: ALC serves as a universal scavenger of free radicals and reduces DNA damage, while NAC is an important substrate for the synthesis of GSH, and ALA is capable of regenerating other antioxidants, including GSH, which plays a critical role in protecting cells from oxidative damage. We developed a simplified sucrose freezing medium for vitrification of human spermatozoa. In this study, we investigated the effect of triple antioxidant supplementation in freezing-thawing medium on both intracellular and extracellular ROS production, and we also evaluated SDF by imaging flow cytometry.
All chemicals were purchased from Sigma-Aldrich Chemicals (Sigma Chemical, St. Louis, MO, USA), unless otherwise stated.
Thirty normozoospermic semen samples from patients who had been referred to the in vitro fertilization clinic of Korat Health Center were included in this study. This study was approved by the Ethics Committee for Research Involving Human Subjects of Suranaree University of Technology, Thailand (EC-63-80). All participants signed an informed consent form before participating in this study. Alcohol drinkers and smokers were excluded from this study, as well as those with a chronic illness or serious systemic disease, genital infection, or varicocele. The semen samples were collected by masturbation after abstinence of 2-7 days and allowed to liquefy for 30 minutes at 37┬║C. Semen analysis was performed according to the guidelines of the World Health Organization [20], and only semen samples exhibiting parameters within the normal ranges were used in the study.
Liquefied semen samples were placed on the top of two layers (40% and 80% fractions) of Sil-Select Stock solution (FertiPro NV, Beemem, Belgium) and centrifuged at 350 ├Śg for 10 minutes to separate immotile and motile sperm from seminal plasma. The pellets were then re-suspended in washing medium consisting of 3 mL of EarleŌĆÖs Balanced Salt Solution (EBSS; Biological Industries, Kibbutz Beit Haemek, Israel) supplemented with 0.3% human serum albumin (Life Global, Guilford, CT, USA), 0.03 M sodium pyruvate, and 10 mM HEPES, and centrifuged at 200 ├Śg for 5 minutes. This washing step was repeated twice. After discarding the supernatant, the final pellet was re-suspended in 500 ┬ĄL of the washing medium and allocated into five aliquots: (1) fresh control, (2) sucrose vitrification (SuV), (3) sucrose vitrification supplemented with triple antioxidants (SuV+3A), (4) the vapor method (Vapor), and (5) the vapor method supplemented with triple antioxidants (Vapor+3A).
The sperm samples were cryopreserved by two different protocols (the SuV and liquid nitrogen vapor methods). In this study, the sucrose vitrification medium was phosphate buffered saline (PBS) solution containing 10% (w/v) bovine serum albumin and 0.5 M sucrose. For the sucrose vitrification method, each sperm sample (100 ┬ĄL/aliquot) was diluted 1:1, with sucrose freezing medium supplemented with 10 ┬ĄM (ALC; Abcam, Cambridge, UK), 10 ┬ĄM NAC, and 5 ┬ĄM ALA (SuV+3A) or without triple antioxidants (SuV). The concentrations of the three antioxidants in this study were based on a previous study by Truong and Gardner [18]. Then, the samples were loaded into 0.25-mL straws and incubated at 4┬░C for 10 minutes. After 10 minutes, the straws were inserted into the holes of a pre-cooled home-made aluminum block, which was previously immersed in liquid nitrogen [21]. The vitrified straws were left on liquid nitrogen for at least 1 week before subsequent experiments.
For the liquid nitrogen vapor method (Vapor), each sperm sample (100 ┬ĄL/aliquot) was diluted with an equal volume of Spermfreeze medium (Fertipro, Beernem, Belgium) supplemented with (Vapor+3A) or without triple antioxidants (Vapor), as described in the previous paragraph. The mixtures were loaded into 0.25-mL straws and incubated at room temperature for 10 minutes. The straws were placed in a horizontal position at a distance of 5ŌĆō7 cm above the level of liquid nitrogen for 15 minutes, and they were directly plunged into liquid nitrogen. The vitrified straws were left on liquid nitrogen for at least 1 week before subsequent experiments.
In the warming steps, the straws were thawed in 25┬░C water, washed in EBSS medium supplemented with or without triple antioxidants, and centrifuged at 200 ├Śg for 3 minutes. After centrifugation, the supernatant was discarded, the final pellet was re-suspended in 100 ┬ĄL of the washing medium, and the sperm parameters were immediately assessed.
The post-thaw samples were immediately assessed for sperm motility and kinematic parameters using a computer-assisted semen analyzer (CASA; HTM IVOS II, Hamilton Thorne Biosciences, Beverly, MA, USA). Progressive motility (%), total motility (%), average path velocity (VAP, ┬Ąm/sec), straight line velocity (VSL, ┬Ąm/sec), curvilinear velocity (VCL, ┬Ąm/sec), amplitude of lateral head displacement (ALH, ┬Ąm), beat-cross frequency (Hz), straightness (STR, [VSL/VAP]├Ś100), and linearity (LIN, [VSL/VCL]├Ś100) were evaluated.
The morphology of sperm was assessed by staining with Diff-Quick (Arnaparn, Nonthaburi, Thailand) and analyzed by an HTM IVOS II CASA equipped with a Dimensions II Strict Morphology software system using KrugerŌĆÖs strict criteria. A total of 200 spermatozoa were analyzed in each slide at ├Ś400 magnification.
Sperm viability was assessed using 0.5% (w/v) eosin-Y dissolved in 0.9% NaCl. A 10-┬ĄL sperm suspension was mixed with 10 ┬ĄL of 0.5% eosin-Y. Then, the mixture was placed on a glass slide and covered with a coverslip. The samples were immediately assessed for sperm viability using a compound microscope (Olympus, Tokyo, Japan). A total of 200 spermatozoa were analyzed in each slide [22]. The spermatozoa were classified as live (unstained heads) or dead sperm (stained red or dark pink heads) and reported as the percentage of live sperm.
The extracellular ROS level was assessed by a chemiluminescence technique, using a Glomax 20/20 luminometer (Turner Biosystems, Sunnyvale, CA, USA). The result was presented as relative light units (RLU) of counted photons per minute or mV/s. Briefly, 10 ┬ĄL of sperm samples from each aliquot were diluted with 400 ┬ĄL of PBS and mixed with 10 ┬ĄL of luminol reagent (5-amino-2,3 dihydro-1,4 phthalazinedione). Then, each sample was measured twice, the average value of RLU/sec was corrected by dividing with the sperm concentration, and the final value of extracellular ROS was expressed in units of RLU/sec/106. A final extracellular ROS value lower than 20 RLU/sec/106 was classified as normal [23].
The intracellular sperm ROS level was evaluated using cell-permeable 2ŌĆÖ7ŌĆÖ-dichlorofluorescein diacetate (DCFH-DA), which was oxidized by the free intracellular H2O2 molecules into green fluorescence dichlorofluorescein (DCF). A total amount of 100 ┬ĄM DCFH-DA and 2.5 ┬ĄM propidium iodide (PI) was separately added to a concentration of 5 ├Ś106 sperm/mL from each sample, followed by incubating at 37oC in 5% CO2 for 10 and 2 minutes, respectively. After incubation, the samples were washed with PBS and analyzed using an imaging flow cytometer (Amnis-Merck, Seattle, WA, USA) equipped with a charge-coupled device (CCD) camera, and a laser operated at 20 mW as a light source. At least 5,000 events were collected for each sample and analyzed by FlowSight (Amnis-Merck). Sperm populations were identified by plotting the forward scatter and side scatter, excluding other debris. Green fluorescence (DCF) was evaluated between 500 and 530 nm, while red fluorescence PI was evaluated between 580 and 630 nm (excitation 488 nm; emission, 530 nm in the FL-2 channel and 632 nm in the FL-5 channel). The percentage of viable DCF-positive cells (DCF+, PIŌĆō) and the mean fluorescence were calculated using image analysis software (IDEAS, Amnis-Merck).
The sperm chromatin structure assay (SCSA) is a flow cytometric assay that relies on the fact that abnormal sperm chromatin is highly susceptible to physical induction of partial DNA denaturation in situ [24]. It measures the intensity of acridine orange (AO) fluorescence using flow cytometry. The SCSA was performed according to the procedure described by Evenson et al. [25], with some modifications. In brief, 100 ┬ĄL of sample from each aliquot was diluted in TNE buffer (0.5 M NaCl, 0.01 M Tris, 0.001 M EDTA, pH 7.4) to a final concentration of 5 ├Ś106 sperm/mL. Then, 200 ┬ĄL of low-pH denaturing solution (0.15 M NaCl, 0.08 N HCl, 0.01% Triton X-100, pH 1.2) was directly added to the diluted sample and incubated for 30 seconds. Then, the sample was stained with 30 ┬ĄL of staining solution (0.2 M NaH2PO4, 1 mM disodium EDTA, 0.15 M NaCl, 0.1 M citric acid monohydrate, pH 6.0) containing 6 ┬Ąg/mL AO and loaded into an imaging flow cytometer (Amnis-Merck) equipped with a CCD camera and a laser operated at 20 mW. The sample was exposed to 488-nm laser light. At least 5,000 events from each sample were collected by FlowSight and analyzed using image analysis software (IDEAS). AO fluoresces green when it binds to native DNA (530┬▒30 nm) and red when it binds to fragmented DNA (>630 nm), as shown in Figure 1.
All data, the mean numbers of sperm motility and kinematics, sperm viability, normal morphology, ROS levels, and percentage of DNA fragmentation were presented as mean┬▒standard error of the mean and compared by one-way analysis of variance (ANOVA). The percentage data were arcsine-transformed to obtain a normal distribution before analysis with one-way ANOVA using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). The differences were compared by the post-hoc FisherŌĆÖs protected least significant difference test. Significant differences were defined as a p-value less than 0.05.
The average age of the 30 donors was 34.5┬▒0.8 years. The semen parameters, including semen volume, concentration, and motility before sperm preparation were 2.8┬▒0.2 mL, 57.5┬▒5.6 ├Ś106 sperm/mL, and 71.7%┬▒2.5%, respectively.
The cryoprotective effects on motility parameters of vitrified spermatozoa are illustrated in Table 1. The total motility after warming was significantly lower in all vitrified sperm groups than in the fresh control group (p<0.05), and a similar phenomenon was also observed for progressive motility (p<0.05). Statistically significant differences were found in some parameters associated with the cryopreservation process, including VAP, VCL, ALH, and STR (p<0.05), while VSL was not significantly different (p>0.05) from the fresh control group. All vitrified sperm groups showed significantly lower viability than the control group (p<0.05). However, no significant differences in terms of post warming sperm morphology were observed among the fresh control and vitrified groups (p>0.05).
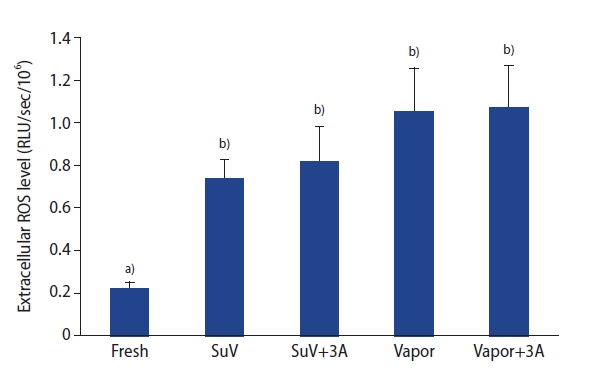
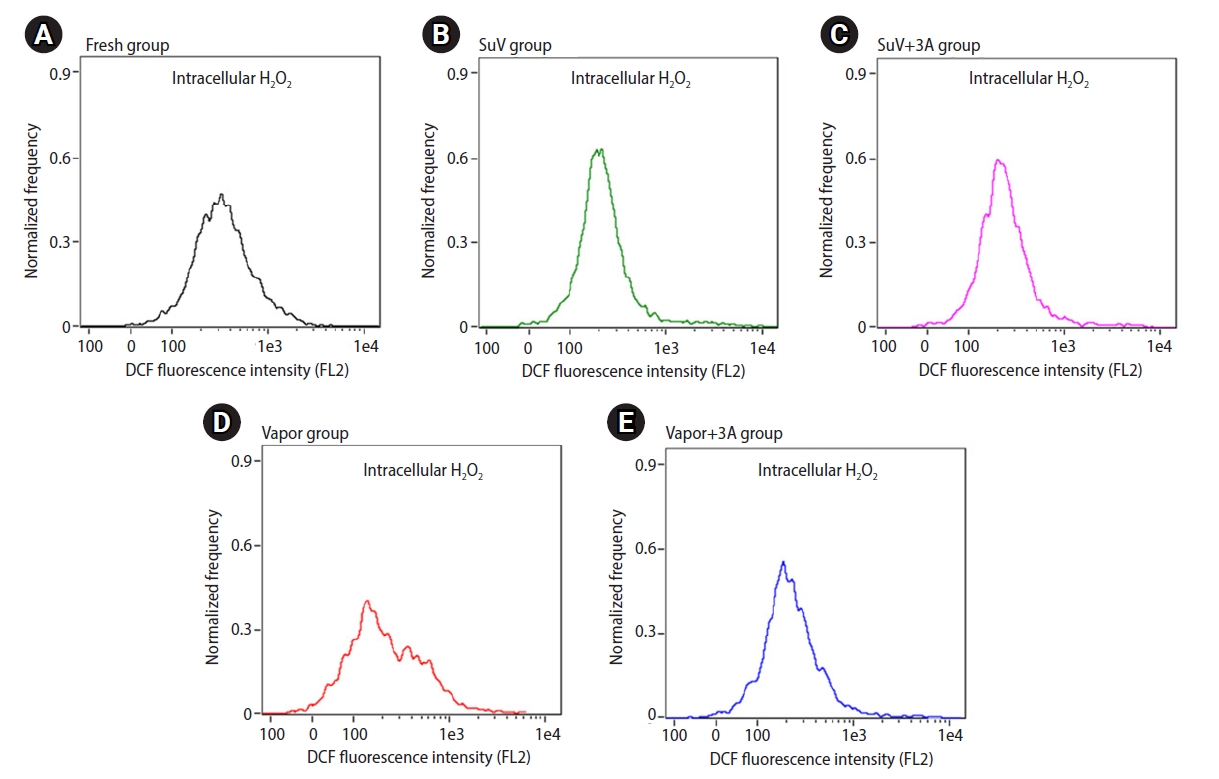
The levels of ROS in the sperm suspension were measured by a chemiluminescence assay. The extracellular ROS levels in sperm suspensions were significantly higher after the freeze-thawed process (SuV, 0.74┬▒0.09; SuV+3A, 0.82┬▒0.16; Vapor, 1.05┬▒0.21; Vapor+3A, 1.07┬▒0.20 RLU/sec/106) than in fresh control group (0.22┬▒0.03 RLU/sec/106, p<0.05) (Figure 2). The mean intensity of DCF fluorescence (intracellular ROS levels) did not significantly differ between the fresh control and vitrified groups (p>0.05) (Figure 3)
To evaluate the protective effect of antioxidants on sperm DNA integrity, the DNA fragmentation rate of spermatozoa was assessed by flow-based SCSA after the freezing-thawing process. As shown in Table 2, the DNA fragmentation rate of spermatozoa did not differ significantly between the fresh control and vitrified groups. However, the DNA fragmentation rate showed a tendency to be higher following the freezing-thawing process in the SuV and Vapor groups than in the fresh control group (p=0.075, p=0.077, respectively). The rate of high DNA stainability (HDS) was significantly higher in the Vapor and Vapor+3A groups than in the fresh control and SuV groups. Supplementation with antioxidants in the freezing and thawing medium had a positive effect on reducing the DNA fragmentation rate, but it was not significant.
Sperm cryopreservation, which is routinely utilized in human ART programs, is closely associated with the use of permeable CPA or a combination of permeable and non-permeable CPAs [26]. However, permeable CPAs are inseparably linked with the problem of toxicity, which damages cell membranes and results in reduced sperm motility and loss of sperm function [27]. Non-permeable CPAs play a supporting role that substantially enhances the effectiveness of permeable CPA. They do not directly penetrate the membrane, resulting in decreased toxicity compared to permeable CPAs [3]. Sperm viability and motility after cryopreservation are important parameters that predict the likelihood of in vitro fertilization [28]. In this study, we demonstrated the feasibility of human sperm vitrification using only sucrose as a non-permeable CPA. The method yielded high recovery rates of viable and motile sperm cells. Our results revealed significantly lower sperm viability and motility in the vitrified groups than in the fresh control group, which is in agreement with earlier studies [29-31]. Cryopreservation has deleterious effects on sperm motility by damaging the plasma membrane and mitochondrial function [29,32]. However, we did not find statistically significant differences in the viability and motility parameters of post-thawed samples between sucrose vitrification and the commercial sperm freezing medium.
Previous studies have focused on other motility parameters as crucial for the prediction of male fertility [33]. Some reports have found a correlation between certain CASA motility parameters (such as ALH, VSL, VCL, and LIN) and human fertility [34]. In addition, ALH values were shown to be a reliable predictor for achieving clinical pregnancy [35]. Researchers also found that STR and LIN values had a significant positive correlation with fertility [34]. In our study, we found a deleterious effect of the freezing-thawing procedure on sperm kinematic parameters, such as VAP, VCL, and ALH. Our motility values are comparable with those of previous reports [36,37]. Furthermore, cryopreservation has a detrimental effect not only on the percentage of total motility, but also on progressive motility. As a result, a decrease in viability and motility values in frozen sperm may affect fertility compared with fresh sperm.
There is evidence that cryopreservation can cause cellular damage by different pathways. The excessive production of ROS is known to play an important role in this regard [29,38]. A large amount of ROS production may cause the accumulation of high levels of peroxides and free radical molecules, which can negatively impact normal sperm function, resulting in loss of sperm motility and viability [27]. In our study, the extracellular ROS levels were significantly higher in all frozen-thawed aliquots. This result is consistent with other research on human sperm cryoinjury [39], which indicated that the extracellular ROS levels increased during cryopreservation by increasing mitochondrial and membrane NADPH oxidase-5 (NOX5) activity. The increased ROS production following cryopreservation may be a result of mechanical damage to the sperm plasma membrane [32,40]. An alternative explanation is that the balance between ROS production and antioxidant scavenging systems is disrupted during the freezing-thawing process.
Recent studies have suggested that the use of antioxidants such as cysteine [41], alpha-tocopherol [42], and GSH [43] may exert beneficial effects on reducing the harmful effects of ROS. A trend now exists for the use of natural antioxidants due to toxicological concerns related to synthetic antioxidants [6,44]. In this study, we used a combination of natural antioxidants (ALC, NAC, and ALA) in the cryopreservation and post-thaw media. Supplementation of these antioxidants could enhance post-thaw motility and viability. However, they had no effect on extracellular ROS after the freezing-thawing process. The relationship between antioxidant supplementation and their beneficial effects remains a controversial topic in the management of cellular oxidative stress. Several studies have reported that supplementation with a combination of antioxidants had no effect on the antioxidants level [45,46], whereas other reports have described positive therapeutic effects [47,48]. It may obscure rather than clarify the discussion of these situations to view the principle of these effects as a clear mechanism.
In our study, the use of triple antioxidants did not decrease the levels of extracellular ROS. Supplementation of NAC (a substrate for the synthesis of GSH), and ALA (stimulator of GSH synthetase) was probably not a good choice because sperm cells, unlike other cells, shed most of their cytoplasm during maturation. As a result, intracytoplasmic enzymatic antioxidant defense mechanisms could be lost or markedly decreased. The findings that SDF had a tendency to decrease without a concomitant reduction in ROS levels could imply that other mechanisms were involved. Although ROS are among the most studied reactive molecules, there are at least three other groups of such species, designated by their reactive heteroatom as reactive nitrogen species, reactive sulfur species, and reactive halogen (chlorine and bromine) species [49]. These endogenous molecular species might not have been detectable as ROS in our detection system. ALC could function as a universal scavenger of reactive species, and thus confer partial protection against DNA damage.
Flow cytometry is a useful tool to identify sperm populations with dysfunctional ability due to intracellular ROS generation [50]. Cryodamage to spermatozoa is likely to be multifactorial mechanisms. In our study, we found that intracellular ROS levels did not differ between the fresh control and frozen-thawed groups. Measuring intracellular ROS using DCF dye is an indirect, non-specific method to determine all the real ROS generated inside sperm cells [51]. This finding was contrary to the levels of extracellular ROS, which significantly increased after cryopreservation by vitrification and vapor freezing method. The low level of intracellular ROS levels detected in this study could perhaps be explained by the principle of the DCFH-DA assay and the unique compartmentalization of sperm cells. To measure intracellular ROS, DCFH-DA must diffuse into viable sperm cells and be deacetylated by cellular esterase in human sperm, forming DCFH. DCFH is a polar molecule that is membrane-nonpermeable and is later oxidized by ROS inside the cells into fluorescent DCF, which can be detected by flow cytometry. Unlike other cells, the sperm nucleus in the head is physically separated from the mitochondria in the midpiece. ROS generated inside the mitochondria react with DCFH and produce DCF that is retained inside the midpiece [52]. The dye could not show fluorescent signals because there was a very scanty amount of mitochondria. Only a small amount of ROS, not neutralized by the sperm antioxidant system, produces weak fluorescent signals. In addition, cellular stress during freezing-thawing procedures has been found to cause impairment of the sperm plasma membrane. ROS molecules (especially H2O2), which were not detected as intracellular ROS, may have passed through the aquaporin pores in the midpiece [53], and were detected as extracellular ROS by the luminol chemiluminescence technique.
Flow-based SCSA is currently the gold standard for DNA fragmentation screening in infertile men to predict fertility outcomes [54]. The Sperm DNA Fragmentation Study Group also recommends that the SCSA, sperm chromatin dispersion, terminal deoxynucleotidyl transferase dUTP nick end labeling, and comet assays are reliable as stand-alone SDF tests, although they may explore slightly different aspects of DNA fragmentation. The results of SCSA are based on the DNA fragmentation index (DFI) or the percent of cells outside the main population (COMP╬▒T), which correspond to sperm cells containing DNA damage. HDS is considered as indicating immature spermatozoa [24]. Earlier research showed that DFI Ōēź30% and HDS Ōēź15% were associated with low fertilization and pregnancy rates [24]. There are many causes of SDF, which may impact male fertility, such as lifestyle factors, infection, varicocele, defective protamination during spermatogenesis, and errors in cryopreservation [55-57].
The present study indicated that vitrification had an adverse effect on SDF during cryopreservation. This result is consistent with previous studies showing that the number of sperm with fragmented DNA was associated with a freezing-thawing procedure [50]. We did not find any relationship between ROS and DNA fragmentation. Besides ROS, other pathways or factors could contribute to SDF. Therefore, the exact mechanisms influencing SDF remain unclear. Further study of these pathways will enhance our understanding of SDF and could provide an effective basis for prevention through antioxidant supplementation. In our study, supplementation with triple antioxidants did not significantly decrease SDF compared to the non-supplemented group. Furthermore, the main limitation of this study was the variation in sperm quality among participants used for these experiments.
In summary, a simplified vitrification medium, consisting of sucrose, compared favorably with the conventional liquid nitrogen vapor freezing protocol. Triple antioxidants in this study, aimed at increasing the activity of the enzymatic antioxidant pathways inside the sperm cytoplasm, did not have significant effects on improving sperm motility, viability, and DNA fragmentation. In future studies, extracellular antioxidants should be considered instead of those that rely on the endogenous enzymatic pathway, as mature sperm contain a scant amount of cytoplasm. The commonly used method of flow cytometric measurements of ROS production based on DCFH-DA is probably inappropriate for sperm because of their unique structure. ROS might not be the only reactive radicals involved in sperm damage after cryopreservation. Clinical outcomes, such as sperm motility, viability, DNA fragmentation, fertilization, and live birth, might be better indicators than ROS production.
Notes
Acknowledgments
The author would like to thank the Korat Health Center for their kind assistance with this study.
Figure┬Ā1.
Determination of human sperm DNA fragmentation by imaging flow cytometry. On the left panel, yellow to red-stained cells indicate DNA fragmentation and green-stained cells indicate intact DNA in sperm, respectively. On the right panel, the sperm DNA fragmentation was evaluated individually by the intensity of acridine orange using imaging flow cytometry. High to moderate DNA fragmentation is shown in red and yellow colors, respectively. Normal to low DNA fragmentation is shown in green. SCSA, sperm chromatin structure assay; HDS, high DNA stainability; DFI, DNA fragmentation index; BF, bright field; AO, acridine orange; Mod, moderate.
Figure┬Ā2.
Comparison of extracellular reactive oxygen species levels between fresh and freeze-thawed spermatozoa supplemented with or without the use of triple antioxidants. ROS, reactive oxygen species; RLU, relative light units; SuV, sucrose vitrification; 3A, triple antioxidants; Vapor, liquid nitrogen vapor. a),b)Bars with different superscripts differ significantly (p<0.05).
Figure┬Ā3.
Quantitative intracellular H2O2 generation was evaluated by the measurement of dichlorofluorescein (DCF) fluorescence intensity using imaging-flow cytometry. Flow-cytometric histograms show the amount of intracellular H2O2 generation H2O2 in sperm. (A) Fresh nonfrozen group, (B) SuV group, (C) SuV+3A group, (D) Vapor group, and (E) Vapor+3A group. Values are presented as mean┬▒standard error of the mean. Significant differences were defined as p-values less than 0.05. SuV, sucrose vitrification; 3A, triple antioxidants; Vapor, liquid nitrogen vapor freezing.
Table┬Ā1.
CASA motility, kinetic parameters, viability, and morphology of fresh and frozen-thawed human spermatozoa supplemented with or without the use of triple antioxidants (n=30)
| Parameter | Fresh control |
Freeze-thawed spermatozoa |
|||
|---|---|---|---|---|---|
| SuV | SuV+3A | Vapor | Vapor+3A | ||
| Motility (%) | 95.3┬▒0.5a) | 73.3┬▒2.0b) | 76.9┬▒1.7b) | 71.7┬▒2.3b) | 74.7┬▒2.1b) |
| Progressive fraction (%) | 91.1┬▒0.9a) | 65.8┬▒2.1b) | 69.4┬▒1.8b) | 64.6┬▒2.5b) | 66.8┬▒2.1b) |
| VAP (┬Ąm/sec) | 61.0┬▒2.1a) | 46.0┬▒1.4c) | 50.0┬▒1.2b) | 50.7┬▒1.2b) | 50.2┬▒1.4b) |
| VSL (┬Ąm/sec) | 39.1┬▒1.9a) | 35.5┬▒1.4a),c) | 39.3┬▒1.2a),b) | 36.2┬▒1.2a) | 37.1┬▒1.1a) |
| VCL (┬Ąm/sec) | 127.5┬▒4.3a) | 94.5┬▒3.0d) | 101.0┬▒2.9c),d) | 111.4┬▒3.1b) | 109.1┬▒3.7b),c) |
| ALH (┬Ąm) | 7.2┬▒0.2a) | 5.2┬▒0.2d) | 5.5┬▒0.2c),d) | 6.1┬▒0.2b),c) | 7.5┬▒1.6a),b) |
| BCF (Hz) | 26.1┬▒0.7a) | 26.7┬▒0.5a),b) | 27.0┬▒0.4a),b) | 27.9┬▒0.6b) | 28.2┬▒0.5b) |
| STR (%)e) | 65.0┬▒1.6a) | 73.6┬▒1.2b) | 74.8┬▒1.0b) | 69.6┬▒0.9c) | 71.7┬▒0.8b),c) |
| LIN (%)f) | 33.2┬▒1.2a) | 38.5┬▒1.1b),c) | 39.5┬▒1.0c) | 34.0┬▒0.8a),d) | 35.6┬▒0.8b),d) |
| Eosin viability (%) | 91.8┬▒2.0a) | 69.4┬▒2.7b) | 74.0┬▒2.5b) | 69.8┬▒2.2b) | 71.3┬▒2.2b) |
| Normality (%) | 16.4┬▒1.5 | 17.2┬▒1.7 | 18.7┬▒1.7 | 20.0┬▒1.6 | 19.7┬▒1.6 |
Values are presented as mean┬▒standard error of the mean.
CASA, computer-assisted sperm analysis; SuV, sucrose vitrification; 3A, triple antioxidants; Vapor, liquid nitrogen vapor; VAP, average path velocity; VSL, straight line velocity; VCL, curvilinear velocity; ALH, amplitude of lateral head displacement; BCF, beat-cross frequency; STR, straightness; LIN, linearity.
Table┬Ā2.
Comparison of DNA fragmentation test between fresh and freeze-thawed spermatozoa with or without triple antioxidant supplementation (n = 30)
| Parameter | Fresh control | Frozen-thawed spermatozoa | |||
|---|---|---|---|---|---|
| SuV | SuV+3A | Vapor | Vapor+3A | ||
| DNA fragmentation (%) | 7.3┬▒1.2 | 15.3┬▒4.1c) | 8.4┬▒1.6 | 14.0┬▒3.0c) | 9.5┬▒1.8 |
| High DNA stainability (%) | 1.2┬▒0.2a) | 1.5┬▒0.2a) | 1.7┬▒0.2a)b) | 2.2┬▒0.3b) | 2.3┬▒0.3b) |
References
2. Alvarez JG, Storey BT. Evidence for increased lipid peroxidative damage and loss of superoxide dismutase activity as a mode of sublethal cryodamage to human sperm during cryopreservation. J Androl 1992;13:232-41.


3. Isachenko V, Isachenko E, Petrunkina AM, Sanchez R. Human spermatozoa vitrified in the absence of permeable cryoprotectants: birth of two healthy babies. Reprod Fertil Dev 2012;24:323-6.


4. Isachenko V, Maettner R, Petrunkina AM, Sterzik K, Mallmann P, Rahimi G, et al. Vitrification of human ICSI/IVF spermatozoa without cryoprotectants: new capillary technology. J Androl 2012;33:462-8.


5. Hernandez-Garcia D, Wood CD, Castro-Obregon S, Covarrubias L. Reactive oxygen species: a radical role in development? Free Radic Biol Med 2010;49:130-43.


6. Agarwal A, Durairajanayagam D, du Plessis SS. Utility of antioxidants during assisted reproductive techniques: an evidence based review. Reprod Biol Endocrinol 2014;12:112.



7. Aitken RJ, De Iuliis GN. On the possible origins of DNA damage in human spermatozoa. Mol Hum Reprod 2010;16:3-13.


8. Banihani S, Agarwal A, Sharma R, Bayachou M. Cryoprotective effect of L-carnitine on motility, vitality and DNA oxidation of human spermatozoa. Andrologia 2014;46:637-41.


9. Takeo T, Horikoshi Y, Nakao S, Sakoh K, Ishizuka Y, Tsutsumi A, et al. Cysteine analogs with a free thiol group promote fertilization by reducing disulfide bonds in the zona pellucida of mice. Biol Reprod 2015;92:90.


10. Shen T, Jiang ZL, Li CJ, Hu XC, Li QW. Effect of alpha-lipoic acid on boar spermatozoa quality during freezing-thawing. Zygote 2016;24:259-65.


11. Mongioi L, Calogero AE, Vicari E, Condorelli RA, Russo GI, Privitera S, et al. The role of carnitine in male infertility. Andrology 2016;4:800-7.


12. Abdelrazik H, Sharma R, Mahfouz R, Agarwal A. L-carnitine decreases DNA damage and improves the in vitro blastocyst development rate in mouse embryos. Fertil Steril 2009;91:589-96.


14. Zeitoun MM, Al-Damegh MA. Effect of nonenzymatic antioxidants on sperm motility and survival relative to free radicals and antioxidant enzymes of chilled-stored ram semen. Open J Anim Sci 2014;5:50-8.


15. Packer L, Witt EH, Tritschler HJ. alpha-Lipoic acid as a biological antioxidant. Free Radic Biol Med 1995;19:227-50.


16. Perera J, Tan JH, Jeevathayaparan S, Chakravarthi S, Haleagrahara N. Neuroprotective effects of alpha lipoic acid on haloperidol-induced oxidative stress in the rat brain. Cell Biosci 2011;1:12.




17. Yun JI, Gong SP, Song YH, Lee ST. Effects of combined antioxidant supplementation on human sperm motility and morphology during sperm manipulation in vitro. Fertil Steril 2013;100:373-8.


18. Truong T, Gardner DK. Antioxidants improve IVF outcome and subsequent embryo development in the mouse. Hum Reprod 2017;32:2404-13.


19. Truong TT, Soh YM, Gardner DK. Antioxidants improve mouse preimplantation embryo development and viability. Hum Reprod 2016;31:1445-54.


20. World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization; 2010.
21. Vutyavanich T, Piromlertamorn W, Nunta S. Rapid freezing versus slow programmable freezing of human spermatozoa. Fertil Steril 2010;93:1921-8.


22. World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed. Cambridge: Cambridge University Press; 1999.
23. Kashou AH, Sharma R, Agarwal A. Assessment of oxidative stress in sperm and semen. Methods Mol Biol 2013;927:351-61.


24. Evenson DP, Larson KL, Jost LK. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl 2002;23:25-43.



25. Evenson DP, Jost LK, Baer RK, Turner TW, Schrader SM. Individuality of DNA denaturation patterns in human sperm as measured by the sperm chromatin structure assay. Reprod Toxicol 1991;5:115-25.


26. Sudagar M, Keivanloo S, Hajibeglou A. Effect of different permeable and non-permeable cryoprotectants on the hatching rate of rainbow trout (Oncorhynchus mykiss) embryos. Aquac Int 2018;26:75-84.


27. Di Santo M, Tarozzi N, Nadalini M, Borini A. Human sperm cryopreservation: update on techniques, effect on DNA integrity, and implications for ART. Adv Urol 2012;2012:854837.

28. Zheng J, Lu Y, Qu X, Wang P, Zhao L, Gao M, et al. Decreased sperm motility retarded ICSI fertilization rate in severe oligozoospermia but good-quality embryo transfer had achieved the prospective clinical outcomes. PLoS One 2016;11:e0163524.



29. Thomson LK, Fleming SD, Aitken RJ, De Iuliis GN, Zieschang JA, Clark AM. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum Reprod 2009;24:2061-70.


30. Isachenko E, Isachenko V, Katkov II, Dessole S, Nawroth F. Vitrification of mammalian spermatozoa in the absence of cryoprotectants: from past practical difficulties to present success. Reprod Biomed Online 2003;6:191-200.


31. Zhou D, Wang XM, Li RX, Wang YZ, Chao YC, Liu ZZ, et al. Improving native human sperm freezing protection by using a modified vitrification method. Asian J Androl 2021;23:91-6.



32. Kumar P, Wang M, Isachenko E, Rahimi G, Mallmann P, Wang W, et al. Unraveling subcellular and ultrastructural changes during vitrification of human spermatozoa: effect of a mitochondria-targeted antioxidant and a permeable cryoprotectant. Front Cell Dev Biol 2021;9:672862.



33. Hirano Y, Shibahara H, Obara H, Suzuki T, Takamizawa S, Yamaguchi C, et al. Relationships between sperm motility characteristics assessed by the computer-aided sperm analysis (CASA) and fertilization rates in vitro. J Assist Reprod Genet 2001;18:213-8.

34. Krause W. Computer-assisted semen analysis systems: comparison with routine evaluation and prognostic value in male fertility and assisted reproduction. Hum Reprod 1995;10 Suppl 1:60-6.


35. Freour T, Jean M, Mirallie S, Dubourdieu S, Barriere P. Computer-assisted sperm analysis (CASA) parameters and their evolution during preparation as predictors of pregnancy in intrauterine insemination with frozen-thawed donor semen cycles. Eur J Obstet Gynecol Reprod Biol 2010;149:186-9.


36. Donnelly ET, McClure N, Lewis SE. Cryopreservation of human semen and prepared sperm: effects on motility parameters and DNA integrity. Fertil Steril 2001;76:892-900.


37. Minaei MB, Barbarestani M, Nekoonam S, Abdolvahabi MA, Takzare N, Asadi MH, et al. Effect of Trolox addition to cryopreservation media on human sperm motility. Iran J Reprod Med 2012;10:99-104.


38. Bansal AK, Bilaspuri GS. Impacts of oxidative stress and antioxidants on semen functions. Vet Med Int 2010;2010:686137.




39. Keshtgar S, Ebrahimi B, Shid-Moosavi SM, Erfani N. NADPH oxidase 5 activation: a novel approach to human sperm cryoinjury. Cell Tissue Bank 2020;21:675-84.



40. Gadea J, Molla M, Selles E, Marco MA, Garcia-Vazquez FA, Gardon JC. Reduced glutathione content in human sperm is decreased after cryopreservation: effect of the addition of reduced glutathione to the freezing and thawing extenders. Cryobiology 2011;62:40-6.


41. Koohestanidehaghi Y, Torkamanpari M, Shirmohamadi Z, Lorian K, Vatankhah M. The effect of cysteine and glutamine on human sperm functional parameters during vitrification. Andrologia 2021;53:e13870.



42. Motemani M, Chamani M, Sharafi M, Masoudi R. Alpha-tocopherol improves frozen-thawed sperm quality by reducing hydrogen peroxide during cryopreservation of bull semen. Span J Agric Res 2017;15:e0401.


43. Shi X, Hu H, Ji G, Liu R, Zhang J, Zhang H, et al. Effects of MTG and GSH on Human Sperm Motility and DNA Integrity during vitrification in the presence of trehalose. Adv Reprod Sci 2019;8:71-81.


44. Adewoyin M, Ibrahim M, Roszaman R, Isa M, Alewi N, Rafa A, et al. Male infertility: the effect of natural antioxidants and phytocompounds on seminal oxidative stress. Diseases 2017;5:9.



45. Anwar AA, Tabri F, Adriani A, Djawad K, Bukhari A, Patellongi I. The effect of glutathione supplementation (L-Glutation, vitamin C, Alpha lipoic acid, and zinc) on total antioxidant status (TAS) level. Int J Med Rev Case Rep 2019;3:195-7.

46. Baumgartner S, Mensink RP, Haenen GR, Bast A, Binder CJ, Bekers O, et al. The effects of vitamin E or lipoic acid supplementation on oxyphytosterols in subjects with elevated oxidative stress: a randomized trial. Sci Rep 2017;7:15288.




47. Abdelhalim M, Qaid HA, Al-Mohy YH, Ghannam MM. The protective roles of vitamin E and ╬▒-lipoic acid against nephrotoxicity, lipid peroxidation, and inflammatory damage induced by gold nanoparticles. Int J Nanomedicine 2020;15:729-34.


48. Khalifa EA, Nabil Ahmed A, Hashem KS, Allah AG. Therapeutic effects of the combination of alpha-lipoic acid (ALA) and coenzyme Q10 (CoQ10) on cisplatin-induced nephrotoxicity. Int J Inflam 2020;2020:5369797.




49. Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol 2004;142:231-55.




50. Cankut S, Dinc T, Cincik M, Ozturk G, Selam B. Evaluation of sperm DNA fragmentation via Halosperm technique and TUNEL assay before and after cryopreservation. Reprod Sci 2019;26:1575-81.



51. Mupfiga C, Fisher D, Kruger T, Henkel R. The relationship between seminal leukocytes, oxidative status in the ejaculate, and apoptotic markers in human spermatozoa. Syst Biol Reprod Med 2013;59:304-11.


52. Aitken RJ, Smith TB, Jobling MS, Baker MA, De Iuliis GN. Oxidative stress and male reproductive health. Asian J Androl 2014;16:31-8.



53. Tafani M, Sansone L, Limana F, Arcangeli T, De Santis E, Polese M, et al. the interplay of reactive oxygen species, hypoxia, inflammation, and sirtuins in cancer initiation and progression. Oxid Med Cell Longev 2016;2016:3907147.




54. Larson-Cook KL, Brannian JD, Hansen KA, Kasperson KM, Aamold ET, Evenson DP. Relationship between the outcomes of assisted reproductive techniques and sperm DNA fragmentation as measured by the sperm chromatin structure assay. Fertil Steril 2003;80:895-902.


55. Yildiz C, Ottaviani P, Law N, Ayearst R, Liu L, McKerlie C. Effects of cryopreservation on sperm quality, nuclear DNA integrity, in vitro fertilization, and in vitro embryo development in the mouse. Reproduction 2007;133:585-95.


- TOOLS







