 |
 |
- Search
| Clin Exp Reprod Med > Volume 48(2); 2021 > Article |
|
Abstract
Objective
Methods
Results
Acknowledgments
Figure 1.
Figure 2.
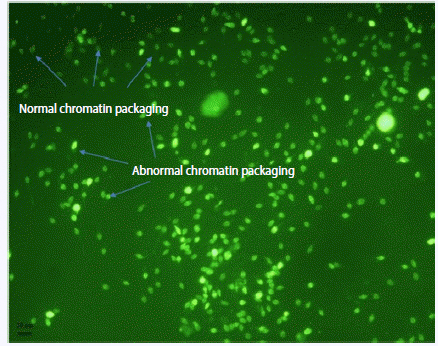
Figure 3.
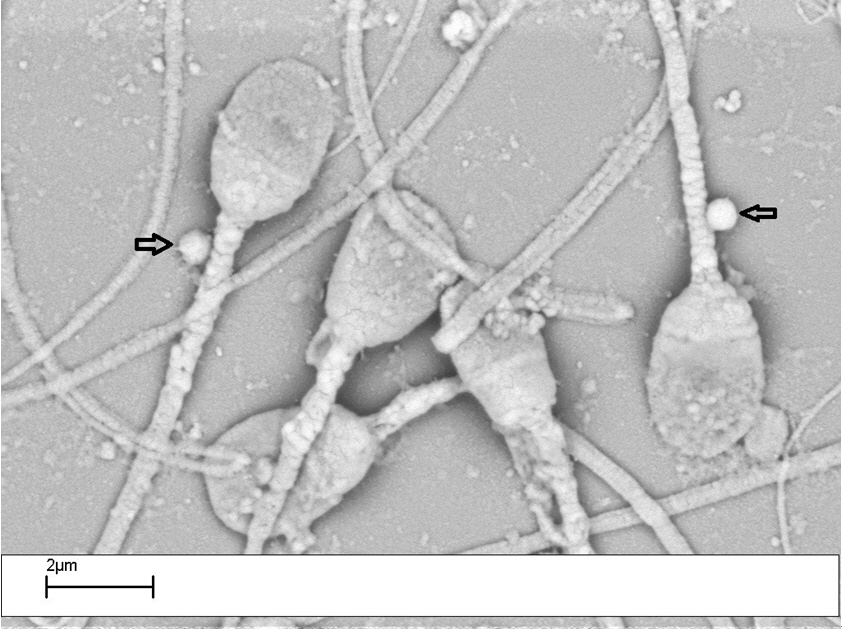
Table 1.
| Variable | No. of patients | CMA3 (%) | Spontaneous AR (%) | AB (%) | TB (%) | Viability (%) |
|---|---|---|---|---|---|---|
| Normozoospermia | ||||||
| Staphylococcus saprophyticus | 41 | 27.2±3.2a) | 20.1±2.2 | 22.6±2.8 | 25.7±2.6 | 68.6±10.8 |
| Escherichia coli | 36 | 20.1±2.2 | 26.2±2.9 | 29.7±2.4a) | 27.8±2.4 | 64.1±9.4b) |
| Nonbacteriospermia | 64 | 14.1±2.6 | 18.1±1.9 | 16.8±1.8 | 20.1±2.9 | 82.2±10.9 |
| Asthenozoospermia | ||||||
| Staphylococcus saprophyticus | 19 | 37.8±2.5b) | 26.4±2.2 | 31.6±2.7 | 25.8±2.6 | 54.9±9.2c) |
| Escherichia coli | 30 | 23.8±2.2 | 29.9±2.3 | 29.1±2.1 | 26.9±2.4 | 53.4±9.1c)> |
| Nonbacteriospermia | 54 | 19.1±1.9 | 19.2±2.4 | 21.8±2.2 | 22.4±2.6 | 79.5±10.0 |
| Teratozoospermia | ||||||
| Staphylococcus saprophyticus | 15 | 36.8±3.6a) | 28.6±2.9 | 36.4±3.3a) | 30.4±3.8 | 59.0±9.5a) |
| Escherichia coli | 35 | 26.6±2.4 | 31.8±3.3 | 34.7±3.6 | 33.6±3.4 | 53.1±9.1b) |
| Nonbacteriospermia | 50 | 22.3±3.2 | 24.3±2.4 | 23.6±2.8 | 26.4±2.6 | 70.8±8.3 |
| Oligoasthenoteratozoospermia | ||||||
| Staphylococcus saprophyticus | 16 | 39.3±3.4 | 27.4±2.2 | 38.7±3.5a) | 35.7±3.6 | 53.4±9.4 |
| Escherichia coli | 25 | 32.4±3.1 | 33.5±2.7a) | 37.4±2.7a) | 37.4±2.5 | 51.9±8.3a) |
| Nonbacteriospermia | 29 | 28.6±2.6 | 20.9±2.2 | 21.6±2.3 | 30.1±2.6 | 61.6±9.1 |
Values are presented as mean±standard deviation. The semen samples infected with S. saprophyticus showed significant differences in terms of sperm quality such as sperm deprotamination (normozoospermia, asthenozoospermia, and teratozoospermia), abnormalities in sperm chromatin condensation (teratozoospermia and oligoasthenoteratozoospermia), and viability (asthenozoospermia and teratozoospermia) in comparison to the control group (non-bacteriospermia). In addition, the semen samples infected with E. coli showed significant differences in sperm quality such as abnormal chromatin condensation (normozoospermia and oligoasthenoteratozoospermia), spontaneous acrosome reaction (oligoasthenoteratozoospermia), and viability (normozoospermia, asthenozoospermia, teratozoospermia, and oligoasthenoteratozoospermia) in comparison to the control group (non-bacteriospermia).
CMA3, chromomycin A3; AR, acrosome reaction; AB, aniline blue; TB, toluidine blue.
Table 2.
| Variable | Fertilization rate (%) | Cleavage rate (%) | Clinical pregnancy rate P/ET (%) | Live birth LB/IE (%) | |
|---|---|---|---|---|---|
| Normozoospermia | |||||
| Staphylococcus saprophyticus | ICSI | 72.2 | 83.4 | 8/14 (57.1) | 4/8 (50.0) |
| IVF | 56.2b) | 78.1 | 8/20 (40.0)b) | 4/8 (50.0) | |
| Escherichia coli | ICSI | 67.2 | 85.9 | 8/17 (47.1)b) | 3/8 (37.5)a) |
| IVF | 67.3a) | 81.6 | 6/13 (46.2)b) | 3/6 (50.0) | |
| Nonbacteriospermia | ICSI | 74.0 | 83.5 | 17/26 (65.4) | 10/17 (58.8) |
| IVF | 79.6 | 85.5 | 22/30 (73.3) | 13/22 (59.1) | |
| Asthenozoospermia | |||||
| Staphylococcus saprophyticus | ICSI | 68.4 | 82.1 | 7/15 (46.7)b) | 3/7 (42.8) |
| Escherichia coli | ICSI | 69.1 | 83.4 | 12/25 (48.0)b) | 4/12 (33.3)a) |
| Nonbacteriospermia | ICSI | 72.7 | 89.3 | 33/49 (67.3) | 18/33 (54.5) |
| Teratozoospermia | |||||
| Staphylococcus saprophyticus | ICSI | 55.9 | 72.8a) | 6/13 (46.2) | 2/6 (33.3)a) |
| Escherichia coli | ICSI | 57.1 | 78.2 | 9/28 (32.1)a) | 3/9 (33.3)a) |
| Nonbacteriospermia | ICSI | 59.1 | 85.0 | 19/41 (46.3) | 10/19 (52.6) |
| Oligoasthenoteratozoospermia | |||||
| Staphylococcus saprophyticus | ICSI | 55.7 | 74.1 | 5/14 (35.7) | 1/5 (20.0)a) |
| Escherichia coli | ICSI | 60.3 | 79.8 | 8/21 (38.1) | 2/8 (25.0) |
| Nonbacteriospermia | ICSI | 58.1 | 76.2 | 10/26 (38.5) | 3/10 (30.0) |
Values are presented as number (%) unless otherwise indicated. The semen samples infected with S. saprophyticus showed significant differences in terms of assisted reproductive outcomes such as the fertilization rate (normozoospermia), embryo cleavage rate (teratozoospermia), clinical pregnancy rate (normozoospermia and asthenozoospermia), and live birth rate (oligozoospermia and oligoasthenoteratozoospermia) in comparison to the control group (non-bacteriospermia). In addition, the semen samples infected with E. coli showed significant differences in the fertilization rate (normozoospermia), pregnancy rate (normozoospermia, asthenozoospermia, and teratozoospermia), and live birth rate (asthenozoospermia and teratozoospermia) in comparison to the control group (non-bacteriospermia).
P, positive cycle; ET, embryo transfer; LB, live birth; IE, implanted embryo; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilization.
References
- TOOLS







