|
 |
- Search
| Clin Exp Reprod Med > Volume 41(4); 2014 > Article |
Abstract
Adenomyosis is a common gynecological disorder characterized by the presence of endometrial glands and stroma deep within the myometrium associated with myometrial hypertrophy and hyperplasia. Focal uterine infarction after IVF-ET in a patient with adenomyosis following biochemical pregnancy has not been previously reported, although it occurs after uterine artery embolization in order to control symptoms caused by fibroids or adenomyosis. We report a case of a nulliparous woman who had uterine adenomyosis presenting with fever, pelvic pain and biochemical abortion after undergoing an IVF-ET procedure and the detection of a slightly elevated serum hCG. Focal uterine infarction was suspected after a pelvic magnetic resonance imaging demonstrated preserved myometrium between the endometrial cavity and inner margin of the necrotic myometrium. This case demonstrates that focal uterine infarction should be considered in the differential diagnosis of acute abdominal pain, vaginal bleeding and infectious signs in women experiencing biochemical abortion after an IVF-ET procedure.
Uterine adenomyosis is a common gynecologic disorder characterized by the presence and growth of heterotopic endometrial or endometrium-like structures in the myometrium, with adjacent smooth muscle hyperplasia and can lead to dysmenorrhea and infertility [1]. The ectopic endometrial tissue induces hypertrophy and hyperplasia of the surrounding myometrium, resulting in diffuse globular enlargement of the uterus analogous to the concentric enlargement of the pregnant uterus. The presenting symptoms include a soft and diffusely enlarged uterus with menorrhagia (40%┬▒50%), dysmenorrhea (10%┬▒30%), metrorrhagia (10%┬▒12%), dyspareunia and dyschezia [2]. Typically the symptoms start one week prior to menstruation. The advised treatment for severe adenomyosis is total hysterectomy, but for patients wishing to preserve their uterus, a minimally invasive alternative procedure, uterine artery embolization (UAE), can be performed. UAE is a nonsurgical alternative for patients with menorrhagia, symptomatic adenomyosis, or symptomatic uterine fibroids [3,4].
Although uterine infarction is a relatively common occurrence after UAE for the treatment of fibroids or adenomyosis [5], uterine infarction after IVF-ET in a patient with adenomyosis is very rare. To our knowledge, this is the first report of uterine infarction after IVF-ET in a patient with uterine adenomyosis in Korea.
A 31-year-old nulliparous woman was admitted to hospital for pelvic pain. She had suffered severe dysmenorrhea, menorrhagia, and pelvic pain due to severe adenomyosis which was diagnosed following a pelvic magnetic resonance imaging (MRI) and clinical examination three years earlier. Her pelvic MRI showed a 9.07├Ś6.89 cm, diffusely enlarged uterus without fibroids. The patient had undergone three unsuccessful cycles of frozen-thawed embryo transfer at an external infertility clinic, due to primary infertility, during the period from 4-7 months before hospital admission. The patient was admitted to hospital for treatment of ovarian hyperstimulation syndrome (OHSS) arising after controlled ovarian hyperstimulation about four months before the initiation of embryo transfer. She had been diagnosed with hyperthyroidism and treated with propylthiouracil from September 2012 onward. A thyroid function test on January 2013 indicated a euthyroid state: triiodothyronine 166 ng/dL (range, 80-200 ng/dL), thyroid stimulating hormone 0.17 ┬ĄIU/mL (range, 0.4-4.1 ┬ĄIU/mL), free thyroxine 1.21 ng/dL (range, 0.80-1.90 mg/dL). The patient underwent another cycle of IVF at a local clinic in February 2013, during which she received follitropin-╬▒ 150 IU from menstrual cycle day 3 to day 12 and chorionic gonadotropin 250 ┬Ąg at menstrual cycle day 13. Ovum pick-up was performed about four weeks before she present to the Emergency Department and embryo transfer three days later. The patient received intramural progesterone 50 mg and was prescribed a vaginal progesterone gel (Crinone) for daily use for 11 days after the embryo transfer.
The patient presented to hospital with vaginal bleeding, fever and abdominal pain on eleven days after embryo transfer, and received antibiotic treatment followed by supportive care at a local clinic for three days. She visited our outpatient clinic due to vaginal bleeding and lower abdominal pain on sixteen days after embryo transfer and was admitted to hospital. C-reactive protein (CRP) was elevated to 7.52 mg/dL and intravenous antibiotic treatment was given. Biochemical abortion was diagnosed on nineteen days following embryo transfer after serum ╬▓-hCG decreased from 161 to 63 mlU/mL in two days. The patient was discharged after symptom improvement, but presented with vaginal bleeding and lower abdominal pain, at a local clinic two days later, where she received hydration. The last day of that month, she presented to the emergency department with high fever (39Ōäā) and, lower abdominal pain. Peripheral blood cell analysis indicated a: white blood cell count of 17.5├Ś103/┬ĄL, an elevated CRP of 24.3 mg/dL, a hemoglobin level of 7.6 g/dL and a hematocrit level of 24.5%. Renal, liver and clotting function tests were within normal limits. Blood culture on admission showed a negative result.
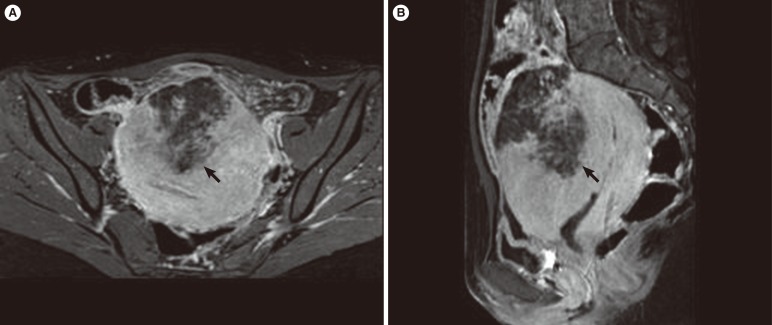
Computed tomography at the Emergency Department revealed a wedge-shaped low attenuation area in the anterior uterine corpus. The radiology department recommended a further contrast-enhanced pelvic MRI study. Focal uterine infarction was suspected, and the pelvic MRI revealed a wedge-shaped, irregularly marginated, non-enhancing area in the anterior uterine wall and the presence of preserved myometrium between the endometrial cavity and the inner margin of the necrotic myometrium (Figure 1). These findings were suggestive of uterine infarction without uterine perforation. Pelvic MRI also revealed peritoneal inflammation.
Antibiotic infusion and red blood cell transfusion were administered. Blood markers investigated to establish the cause of infarction, including antiphospholipid immunoglobulin G (IgG) and IgM, protein C activation, protein S, and plasminogen, were all within normal ranges. Five days after hospital admission, pelvic pain improved and CRP decreased to 6.08 mg/dL, and the patient was discharged. Two months later, pelvic MRI showed interval regression of the previously noted possible focal uterine infarction site with a remarkably improved blood flow (Figure 2). The patient still had dysmenorrhea and menorrhagia but abdominal pain had improved significantly.
Adenomyosis uteri is a pathological entity characterized by the presence of endometrial glands and stroma embedded within the myometrium, but without apparent contact with the endo-myometrial junction [6]. Uterine adenomyosis is relatively frequent, affecting 1% of females; its diagnosis is more often made in multiparous patients, in their fourth and fifth decade of life, and for this reason, infertility is not frequently concurrent with adenomyosis. However, given the trend for first pregnancies to be delayed until women are in their thirties or forties, adenomyosis uteri becoming a more frequently considered diagnosis [2]. Pregnancy resulting from assisted reproductive technology (ART) is possible in patients with ademoyosis; however, spontaneous abortion, including biochemical abortion, still occurs in these patients.
Uterine infarction after IVF-ET in a patient with adenomyosis has not previously been reported. Uterine infarction has been associated with UAE for symptom control of fibroids or adenomyosis [7]. The objective of UAE is to initiate tumor infarction resulting in substantial reduction of the uterine and tumor volumes [8].
It is possible to speculate on the potential mechanisms of occurrence of uterine infarction. First, when undergoing IVF-ET, the risk of thrombosis is increased, as in the cases of pregnancy and hyperthyroidism, with which the patient also presented [9,10]. The precise mechanisms by which the OHSS and exogenous hormonal stimulation used in ART induce thromboembolic events are still uncertain. However, vascular endothelial growth factor secreted during OHSS, high estradiol concentrations, and blood hyperviscosity play a prominent part in inducing a prothrombotic state [11]. Hansen et al. [12] reported that the overall venous thrombosis incidence rate during IVF pregnancies was three times higher than in reference pregnancies. An elevated CRP can activate the coagulation system, following which microthrombosis formation, in the uterine myometrium, could cause necrosis of the myometrium and lead to focal uterine infarction. Second, the disruption of the endometrial-myometrial interface also disrupts the organization of myometrial muscle fibers and may also disrupt normal contractility of the subendometrium [2]. Uterine adenomyosis with abnormal contractility and the subsequent abortion caused massive vaginal bleeding which may have led to uterine ischemia, and the resulting uterine infarction. Third, thyroid dysfunction modifies the balance between coagulation and fibrosis. Clinical hyperthyroidism is generally accompanied by a hypercoagulable state, which may lead to microthrombosis of the uterine myometrium [13]. Last, fever and the elevated CRP suggested that infection could have complicated the ischemic necrosis after uterine infarction [14].
The patient we describe here presented with abnormal vaginal bleeding and pelvic pain. Uterine sarcoma also presents with such symptoms. However, there were no clinical features such as rapid tumor growth, extrauterine extension or metastases [15,16], leading to the conclusion that the possibility of uterine sarcoma was very small. A follow-up MRI three months later showed only traces of uterine infarction lesion and adenomyosis.
In conclusion, we experienced a rare case of uterine adenomyosis that developed into uterine infarction after IVF-ET and biochemical pregnancy. This case demonstrates that uterine infarction should be considered in the differential diagnosis of acute abdominal pain, vaginal bleeding and infectious signs in women with biochemical abortion after IVF-ET, with a history of adenomyosis and hyperthyroidism. The insertion of a hormonal intrauterine device could be considered another option for treating the symptoms of adenomyosis in cases presenting with focal uterine infarction dysmenorrhea and menorrhagia. In addition, the surgical management of focal uterine infarction, may be considered in the management of uterine infarction after IVF-ET in patients with uterine adenomyosis if future fertility is not desired. Finally, it is important to note that the previous infarction site might be a potential risk site for uterine rupture in a future pregnancy.
References
1. Valentini AL, Speca S, Gui B, Soglia G, Micco M, Bonomo L. Adenomyosis: from the sign to the diagnosis. Imaging, diagnostic pitfalls and differential diagnosis: a pictorial review. Radiol Med 2011;116:1267-1287.PMID: 21892720.


2. Devlieger R, D'Hooghe T, Timmerman D. Uterine adenomyosis in the infertility clinic. Hum Reprod Update 2003;9:139-147.PMID: 12751776.


3. Bradley LD. Uterine fibroid embolization: a viable alternative to hysterectomy. Am J Obstet Gynecol 2009;201:127-135.PMID: 19646564.


4. Kim MD, Kim S, Kim NK, Lee MH, Ahn EH, Kim HJ, et al. Long-term results of uterine artery embolization for symptomatic adenomyosis. AJR Am J Roentgenol 2007;188:176-181.PMID: 17179361.


5. Nijenhuis RJ, Smeets AJ, Morpurgo M, Boekkooi PF, Reuwer PJ, Smink M, et al. Uterine artery embolisation for symptomatic adenomyosis with polyzene f-coated hydrogel microspheres: three-year clinical follow-up using UFS-QoL Questionnaire. Cardiovasc Intervent Radiol 2014;4 02 [Epub]. http://doi.dx.org.10.1007/s00270-014-0878-1

6. Bergholt T, Eriksen L, Berendt N, Jacobsen M, Hertz JB. Prevalence and risk factors of adenomyosis at hysterectomy. Hum Reprod 2001;16:2418-2421.PMID: 11679531.


7. Verma SK, Gonsalves CF, Baltarowich OH, Mitchell DG, Lev-Toaff AS, Bergin D. Spectrum of imaging findings on MRI and CT after uterine artery embolization. Abdom Imaging 2010;35:118-128.PMID: 19048334.


8. Lohle PN, De Vries J, Klazen CA, Boekkooi PF, Vervest HA, Smeets AJ, et al. Uterine artery embolization for symptomatic adenomyosis with or without uterine leiomyomas with the use of calibrated tris-acryl gelatin microspheres: midterm clinical and MR imaging follow-up. J Vasc Interv Radiol 2007;18:835-841.PMID: 17609441.


9. Levin I, Gamzu R, Pauzner D, Rogowski O, Shapira I, Maslovitz S, et al. Elevated levels of CRP in ovarian hyperstimulation syndrome: an unrecognised potential hazard? BJOG 2005;112:952-955.PMID: 15957998.


10. Brenner B. Thrombophilia in pregnancy and its role in abortion. Womens Health (Lond Engl) 2005;1:35-38.PMID: 19803944.

11. Alatri A, Tribout B, Gencer B, Calanca L, Mazzolai L. Thrombotic risk in assisted reproductive technology. Rev Med Suisse 2011;7:357-360.PMID: 21416715.

12. Hansen AT, Kesmodel US, Juul S, Hvas AM. Increased venous thrombosis incidence in pregnancies after in vitro fertilization. Hum Reprod 2014;29:611-617.PMID: 24399508.


13. Targher G, Pichiri I, Zoppini G, Bonora E, Chonchol M. Hemostatic and fibrinolytic abnormalities in endocrine diseases: a narrative review. Semin Thromb Hemost 2009;35:605-612.PMID: 20013527.


14. Sabatini L, Atiomo W, Magos A. Successful myomectomy following infected ischaemic necrosis of uterine fibroids after uterine artery embolisation. BJOG 2003;110:704-706.PMID: 12842064.


15. Perri T, Korach J, Sadetzki S, Oberman B, Fridman E, Ben-Baruch G. Uterine leiomyosarcoma: does the primary surgical procedure matter? Int J Gynecol Cancer 2009;19:257-260.PMID: 19396005.