|
 |
- Search
| Clin Exp Reprod Med > Volume 41(1); 2014 > Article |
Abstract
Objective
To investigate the association of individual follicular fluid (FF) leptin and adiponectin levels with the quality of the corresponding oocyte and embryo.
Methods
We prospectively enrolled 67 women who underwent controlled ovarian hyperstimulation with 89 FF samples. FF and the corresponding oocyte was obtained from a single dominant preovulatory follicle at the time of oocyte retrieval. Concentrations of leptin and adiponectin were measured by enzyme-linked immunosorbent assay in an individual follicle. The oocyte quality, fertilization rate, and corresponding embryo development were assessed.
Results
The FF level of leptin was significantly associated with body mass index (r=0.334, p<0.01). The FF adiponectin level was significantly higher in the normal fertilization group than the abnormal fertilization group (p=0.009) in the non-obese women. A lower FF leptin level was associated with a trend toward mature oocytes, normal fertilization, and good embryo quality, although these relationships were not statistically significant. The leptin:adiponectin ratio of FF did not differ significantly according to oocyte and embryo quality. The quality of the oocyte and embryo was not associated with the FF leptin level tertile. However, the normal fertilization rate was positively associated with FF adiponectin level tertile. There was a trend towards improved oocytes and normal fertilization rates with the lowest tertile of the FF leptin:adiponectin ratio, but this difference was not statistically significant.
Currently, the associations of obesity with poor reproductive outcomes such as infertility, lower mature oocyte yield, and lower number of cryopreservation cycles are well known [1,2,3]. A few studies have provided strong evidence that obese women need higher doses of exogenous gonadotropins and have a higher IVF cycle cancelation rate and significantly lower live birth rates after IVF, as well as higher miscarriage rates [2,3,4,5,6,7]. The essential pathophysiology of the impact of increased body mass index (BMI) on reproduction involves disturbances in the hypothalamic-pituitary axis and menstrual cycle alterations leading to anovulation [8,9]. However, studies have shown that even in women who are ovulating regularly, overweight correlates with reduced conception rates [10,11], suggesting that obesity affects critical peri-conception events, such as oocyte and/or embryo quality. Although the mechanism linking obesity to poor reproductive outcomes (oocyte maturity and/or embryo quality) is not well understood, various adipocytokines are thought to be involved [7], and they could affect ovarian intrafollicular alterations at multiple cellular levels including steroidogenesis, metabolic, and inflammatory pathways [12].
White adipose tissue has been recognized as an endocrine organ and an important source of adipocytokines [13,14,15]; two representative members of adipocytokines are leptin and adiponectin, which partly mediate the effects of adiposity on reproduction [16]. Leptin concentrations are directly correlated with absolute fat mass [17]; in contrast, serum concentrations of adiponectin, which is expressed exclusively by adipocytes, are inversely correlated with obesity [18]. In particular, inappropriate secretion of adipocytokines by an excessive volume of white adipose tissue seems to participate in obesity-related pathologic processes.
Leptin has been the focus of numerous studies since its discovery. These studies have consistently demonstrated a positive association between leptin levels and body fat mass [19]. As expected, many studies have demonstrated adverse effects of leptin on IVF outcomes, including inhibition of ovarian follicular development and steroidogenesis [20,21]. However, some have reported no adverse effects [22,23]. Therefore, up to the present, the research community has failed to reach consensus on this issue. In contrast, adiponectin levels have a negative association with body fat mass and the opposite effect of leptin on reproduction. However, the role of adiponectin in human oocyte quality and early embryo development remains in question because of the low level of adiponectin expression by human granulosa cells. Furthermore, very little information on adiponectin in the field of infertility is available in the literature to date.
The ratio of leptin to adiponectin (L:A ratio) has been reported to represent a better indicator of obesity, insulin resistance, and metabolic syndrome than either component alone, in particular in the female population. Recently, Li et al. [16] characterized for the first time the L:A ratio in the serum and follicular fluid (FF) of reproductive aged women in the setting of ovarian stimulation. This study demonstrated that both FF adipocytokines were associated, leptin positively and adiponectin negatively, with successful cleavage as well as viable embryo morphology. Consequently, the overall relationship between the FF L:A ratio and embryo development was positive [16]. However, as the authors observed, these results were counterintuitive and thus difficult to accept because, despite a lack of consensus, many studies have reported the negative impact of FF leptin and positive impact of FF adiponectin on embryology parameters.
Therefore, the present study aimed to investigate the association of FF leptin and adiponectin levels and the L:A ratio with embryo development, presumably by modulating oocyte quality once again. In order to improve accuracy, we aspirated FF and the oocyte from a single dominant follicle in each of the ovaries. We then analyzed the relationship of the FF leptin and adiponectin levels and the L:A ratio with the corresponding oocyte quality, fertilization, and embryo quality.
A total of 67 infertile women undergoing controlled ovarian hyperstimulation (COH) for IVF between March 2007 and July 2011 were included in this study. The cycle was included when at least one oocyte was obtained and the corresponding FF from a single preovulatory follicle was available. Patients with polycystic ovary syndrome, severe endometriosis, hypothalamic amenorrhea, and a history of ovarian surgery were excluded. Informed consent from each patient was obtained before the ovarian stimulation. The patients were between 27 and 44 years old with the following diagnoses leading to IVF: tubal factor (n=17), male factor (n=9), uterine factor (n=9), ovarian factor (n=7), a combination of factors (n=5), unexplained infertility, or another factor (n=20). Concentrations of serum FSH, E2, and anti-Müllerian hormone (AMH) on day 3 of a spontaneous cycle were measured. Height and weight were measured within three months from the start day of COH. In order to use stored FF samples, approval from the Institutional Review Board of Seoul National University Bundang Hospital was obtained.
Ovarian stimulation was performed with recombinant FSH (rFSH, Gonal-F, Merck-Serono, Darmstadt, Germany) and human menopausal gonadotropin (Menopur, Ferring, Saint-Prex, Switzerland), the dose of which was adjusted individually based on the follicular response. Pituitary down-regulation was achieved by the GnRH agonist long protocol or GnRH antagonist protocol. For the GnRH agonist long protocol, 0.1 mg triptorelin acetate (Decapeptyl, Ferring) was injected daily in the mid-luteal phase of the previous menstrual cycle. After pituitary down-regulation, gonadotropin was started on menstrual day 2 or 3. The initial dose of gonadotropin was fixed for the first 4 or 5 days, followed by adjustment on the basis of individual follicular growth until triggering. For the GnRH antagonist protocol, gonadotropin was started on menstrual day 2 or 3. When the leading follicle reached a diameter of 14 mm, 0.25 mg of GnRH antagonist (cetrorelix, Cetrotide; Merck-Serono) was added daily until triggering. When the leading follicle reached a mean diameter of 18 mm or two follicles or more reached a diameter of 17 mm, 250 µg of recombinant hCG (Ovidrel, Merck-Serono) was administered subcutaneously 36 hours before transvaginal oocyte retrieval. Up to 3 embryos were transferred 3 days after oocyte retrieval. The luteal phase was supported with 50 mg of intramuscular progesterone in oil (Progest, Samil, Seoul, Korea) or 8% progesterone gel (Crinone, Merck-Serono) daily, initially for 14 days starting on the day of oocyte retrieval and continuing for another 6 to 8 weeks in cases where a pregnancy was achieved.
At the time of oocyte retrieval, the single dominant follicle (>17 mm in diameter) was punctured. The FF was aspirated into an empty bottle without any media. If one intact oocyte was present and the FF was not contaminated by fresh blood, the FF was isolated. To prevent contamination with other follicles, a different needle was used for the ovary on each side. After the needle was withdrawn, it was flushed to obtain any oocytes trapped in the dead space of the collection needle. The FF and oocyte were obtained from only one follicle for each ovary. Each FF sample was centrifuged immediately at 1,300 g for 10 minutes and the clear supernatants stored at -20℃ until assayed, and each corresponding oocyte was isolated and evaluated.
The preparation of the sperm and oocytes, fertilization, and subsequent culture of the embryo were performed as previously reported [24]. Briefly, the oocytes were placed in insemination medium (Sydney IVF Fertilization Medium, Cook Women's Health, Spencer, IN, USA) 1 to 4 hours before insemination with 50,000 to 500,000 motile spermatozoa per milliliter of medium. If fertilization had failed in previous IVF cycles or the cause of infertility was a male factor, ICSI was performed. Fertilization was assessed 16 to 18 hours after insemination by verifying the presence of two distinct pronuclei and a second polar body. The fertilized oocytes were maintained in culture medium (Sydney IVF cleavage medium).
The oocytes and embryos corresponding to the study follicles were cultured individually in separate dishes. The nuclear maturity of the oocyte was determined before ICSI. Embryologists classified each oocyte based on a morphological analysis: the immature stage if a germinal vesicle was visible, intermature stage if neither the germinal vesicle nor first polar body were visible, and mature stage if the first polar body was visible in the perivitelline space. An oocyte was labeled postmature if it was fractured at retrieval. After incubation with sperm, oocyte was classified as normal fertilization (two pronuclei), failed fertilization (no evidence of sperm penetration), or abnormal fertilization (polyspermy and oocytes with only one pronucleus). On day 3, embryonic morphologic development was assessed as four grades (I-IV) on culture according to the regularity of blastomeres, the percentage of anulceate fragments, and all dysmorphic characteristics of the embryos: 1) grade I, 0% anucleate fragments, regularity of blastomeres, and no apparent morphologic abnormalities; 2) grade II, <20% anucleate fragments, regularity of blastomeres, and no apparent morphologic abnormalities; 3) grade III, 20% to 50% anucleate fragments, irregularity of blastomeres, and no apparent morphologic abnormalities; and 4) grade IV, ≥50% anucleate fragmentation, irregularity of blastomeres, and apparent morphologic abnormalities. Good quality embryos were defined as grade I or II embryos with at least six blastomeres on day 3 after fertilization. Poor quality embryos were defined as grade IV. The embryo score was calculated by the embryo grade multiplied by the number of blastomeres.
FF leptin and adiponectin levels were measured using commercial sandwich enzyme immunoassay kits (Invitrogen Co., Carlsbad, CA, USA) according to the manufacturer's protocol. The assay sensitivity of leptin and adiponectin was 3.5 pg/mL and 100 pg/mL, respectively. The intra- and inter-assay coefficient of variance (CV) for the leptin assay was 3.57% at 425.77 pg/mL and 4.6% at 431.93 pg/mL, respectively; 3 samples were assayed in replicates of 16 to determine intra-assay precision and 40 times in multiple assays to determine inter-assay precision. The intra- and inter-assay CV for the adiponectin assay were 3.49% at 9.91 pg/mL and 4.37% at 11.55 pg/mL, respectively; 4 samples were tested 5 times to assess intra- and inter-assay precision. The L:A ratio was calculated using the leptin and adiponectin levels.
The study subjects were divided into three groups according to the FF levels of leptin and adiponectin, and the L:A ratio. The FF leptin levels were classified into tertiles: the 33rd percentile and below (low group, leptin<9.07 ng/mL), between the 34th and 66th percentile (intermediate group, leptin=9.07-13.13 ng/mL) or the 67th percentile and above (high group, leptin>13.13 ng/mL) of measurements. For the adiponectin levels, the tertiles were as follows: low group, adiponectin<2.45 µg/mL; intermediate group, adiponectin=2.45-5.20 µg/mL; and high group, adiponectin>5.20 µg/mL. The resulting FF L:A ratio was classified as low <0.002; intermediate=0.002-0.008; or high >0.008.
Statistical analysis was performed using PASW statistics ver. 18 (SPSS Inc., Chicago, IL, USA). Correlation among the age, BMI, serum levels of FSH, E2, AMH, FF levels of leptin, adiponectin, and the L:A ratio were evaluated with Spearman's rank correlation analysis. Mann-Whitney and Kruskal Wallis tests were used to compare continuous variables and Fisher's exact test was used for categorical variables. The correlation between the embryo quality score and FF leptin and adiponectin levels and the L:A ratio was evaluated with Spearman's rank correlation analysis. A p-value of <0.05 was considered statistically significant.
A total of 89 FF and oocyte samples from 67 patients were studied. The mean age of the patients was 35.0±3.4 years with a mean BMI of 22.7±3.1 kg/m2. The mean baseline FSH, E2, and AMH were 6.0±2.4 mIU/mL, 32.3±31.9 pg/mL, and 2.4±1.1 ng/mL, respectively. The mean FF leptin and adiponectin levels were 15.9±10.3 ng/mL and 3.9±2.1 µg/mL, respectively. The mean FF L:A ratio was 0.0135±0.0625. The proportion of mature oocytes was 92.1% (82/89) and that of cases of normal fertilization was 80.9% (72/89). The proportion of good quality embryos was 47.3% (35/74) on day 3.
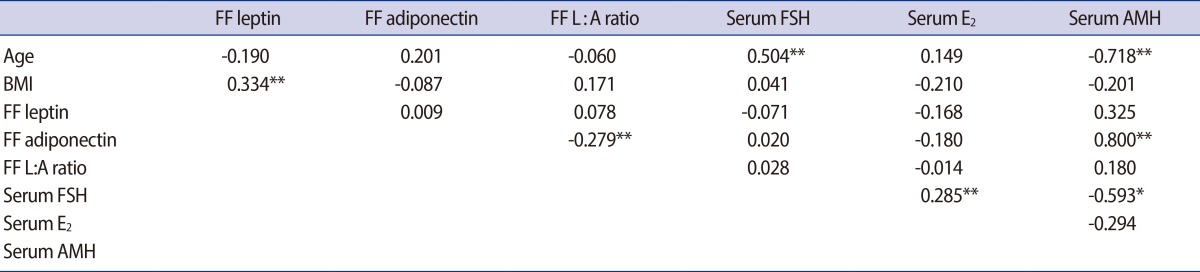
The correlations among age, BMI, serum hormones (FSH, E2, and AMH), FF adipocytokine levels, and the L:A ratio are shown in Table 1. As expected, the FF leptin level was positively correlated with BMI (r=0.334, p<0.01). On the other hand, the FF adiponectin level and the FF L:A ratio were correlated neither with BMI nor with the FF leptin level. The FF L:A ratio, representing the combined effects of FF leptin and adiponectin, had a negative correlation with FF adiponectin (r=-0.279, p<0.01). The serum hormone levels, including FSH and E2, correlated neither with FF adipocytokines nor with the FF L:A ratio. The serum FSH and AMH level were correlated positively with age (r=0.504, p=0.01) and negatively (r=-0.718, p<0.01) with age, respectively.
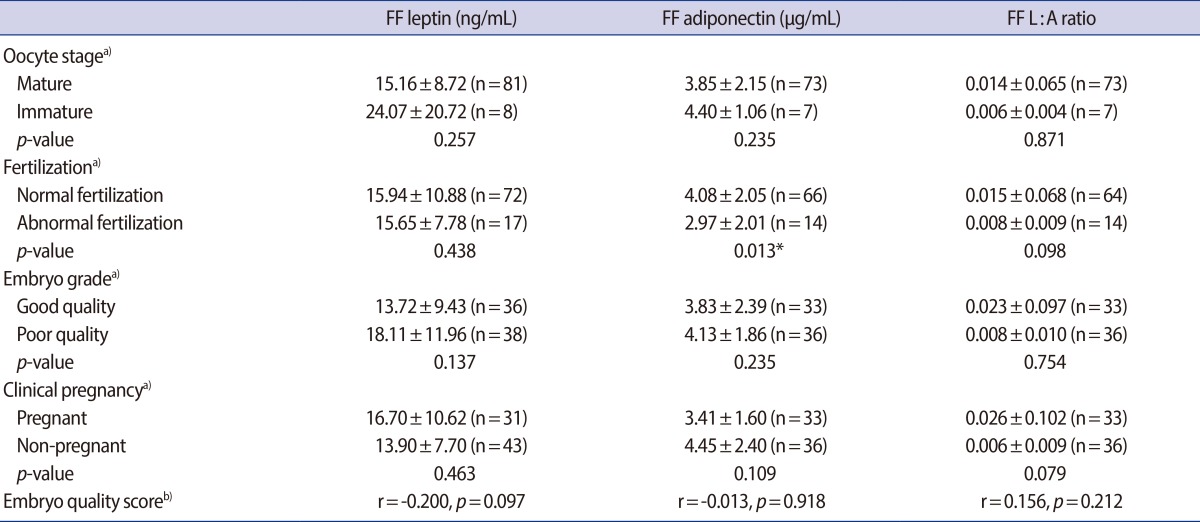
The FF leptin level was lower in the mature oocyte group, and in the good quality embryo group compared with the immature oocyte and poor quality embryo group, respectively, although these differences were not statistically significant (Table 2). There was no difference in the FF leptin level according to fertilization success. The FF adiponectin level was significantly higher in the normal fertilization group than the abnormal group.
However, the FF adiponectin level showed no significant difference according to oocyte maturity and embryo quality. The FF L:A ratio showed no significant differences according to oocyte maturation, fertilization, or embryo quality.
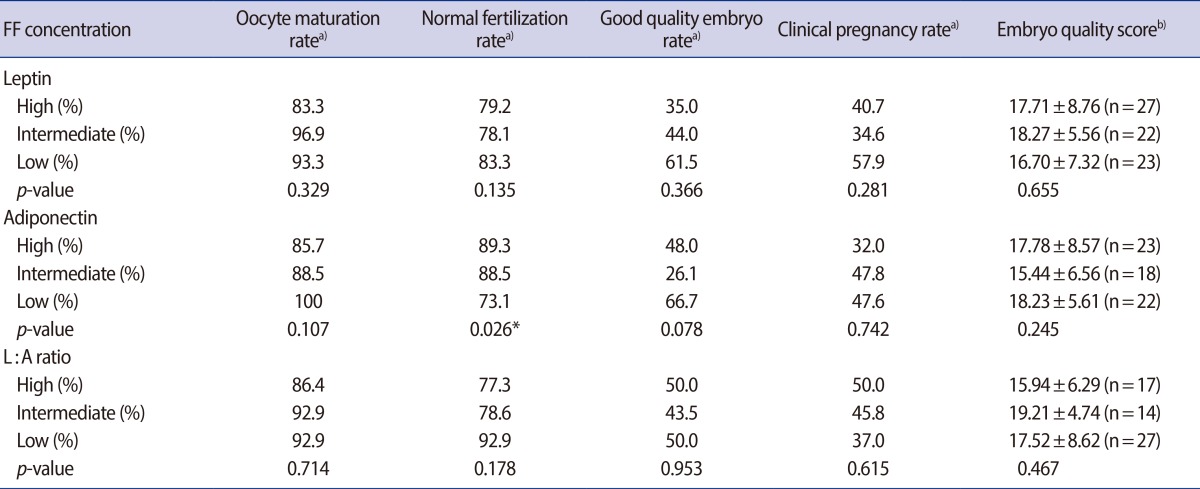
Oocyte and embryo outcomes based on low, intermediate, and high FF adipocytokine levels and L:A ratio tertile are shown in Table 3. There was a trend towards improved oocyte maturation/embryo outcomes with a reduced FF leptin level and lower L:A ratio tertile, although the relationship was not statistically significant. In contrast, increasing FF adiponection was positively associated with successful fertilization (χ2=6.21, p=0.078).
In the present study, we demonstrated that the FF adiponectin level was significantly related to the normal fertilization rate. FF leptin levels and the L:A ratio showed no significant association with oocyte maturation, fertilization, or embryo quality in contrast with our expectations. However, increasing the FF leptin level tended to be associated with adverse outcomes for oocyte maturation and embryo quality. Reproductive function of women could be alternated following severe changes in weight and nutritional status. Leptin is mandatory for normal reproductive function. It acts at multiple levels of the reproductive tissues such as the Hypothalamic-pituitary-ovarian axis [25]. The hypothalamus-pituitary axis probably is not influenced by high circulating levels of leptin because the blood-brain barrier prevents excess concentrations of leptin binding to hypothalamic receptors. It is considered to be a possible regulator of many reproductive functions including steroidogenesis and gametogenesis in the ovary. Since the white adipose tissue has been recognized as an endocrine organ [26], many authors have studied the association of adipocytokines derived from white adipose tissue including leptin and adiponectin with IVF outcomes in terms of oocyte quality, embryo quality, and the fertilization.
There is some evidence that leptin stimulates oocyte maturation or has a beneficial effect on mice, porcine, and bovine oocyte quality [27,28,29,30]. In addition, leptin administration with gonadotropins during superovulation increased the ovarian response and developmental competence of mouse oocytes [31]. In spite of observations of the positive effects of leptin on oocytes in several animal studies, the effect of leptin on human reproductive organs is still debated. Barroso et al. [21] reported that FF levels of leptin correlated negatively with embryo quality in IVF cycles, and several investigators reported that high levels of either serum or intrafollicular leptin are associated with lower pregnancy rates in IVF cycles [20,32,33]. On the other hand,
Welt et al. [22] demonstrated that leptin and the soluble leptin receptor are highly interrelated with each other and with other intrafollicular hormones, but not with markers of oocyte quality, fertilization, or embryo grade. It was also reported that the FF leptin levels did not correlate with the number of oocytes, the fertilization rate, or the embryo quality [23]. Most recently, Li et al. [16] reported that increasing FF leptin tertiles show a trend towards improved IVF outcomes. Our results also showed that FF leptin levels showed a negative trend relative to oocyte and embryo quality. This could be based on species-specific differences and different methodology. We have measured leptin concentrations in individual follicles, whereas others pooled FFs. Pooled FFs are used to negate the variation in leptin concentrations in individual follicles as well as to be consistent with pooled embryo transfer, ovarian response, and pregnancy outcome. In previous studies, it has not always been clearly stated what kind of follicles the fluid has been drawn from.
Regarding adiponectin, however, the majority of studies have examined animal models and few have investigated outcomes after IVF/ICSI. There is also evidence that the expression of adiponectin and its receptors is regulated during follicular and oocyte growth and during early embryo development and implantation [34,35,36]. The women undergoing assisted reproductive treatments showed that the serum level of adiponectin was positively correlated with the average number of oocytes retrieved from each patient. Few controlled studies have evaluated the effect of adiponectin during oocyte maturation and embryo culture in vitro. Recombinant adiponectin (30 µg/mL) enhanced nuclear maturation of porcine oocytes [37], and adiponectin (10 µg/mL) had beneficial effects on in vitro mouse embryo development [36]. Richards et al. [38] reported that the addition of adiponectin to in vitro maturation media might improve the developmental competence of in vitro matured oocytes in human infertility care. Another effect of adiponectin is on embryo implantation. The adiponectin system was more abundantly expressed in human endometrium during the luteal period, which corresponds to the embryo implantation period [39]. The protein levels of adiponectin, AdipoR1, and AdipoR2 were found to be higher in the endometrium at the sites of embryo implantation compared to the interimplantation sites [35]. Adiponectin has anti-inflammatory and insulin sensitizing properties, and is negatively associated with obesity [34]. Our study also showed positive results, in that a higher FF adiponectin level was significantly associated with normal fertilization. However, others have reported that an increasing FF adiponectin tertile was negatively associated with embryo quality including viable cleavage morphology, successful blastulation, and viable blastocyst morphology [16]. Due to conflicting results, additional studies are necessary to further distinguish the mechanisms by which adiponectin affects oocytes, embryo development, and implantation.
Accurate and reliable research on the relationship of oocyte and embryo quality with adipocytokines was important. To determine the precise relationships between oocyte/embryo quality and adipocytokines, we analyzed the FF sample as a microenvironment directly affecting the quality of the embryo. We found that the FF derived from a single follicle will most accurately reflect the quality of the oocyte from a single follicle. This is the first study to analyze the FF and corresponding oocyte derived from a single dominant follicle for improving the accuracy of the effect of leptin and adiponectin on oocyte quality. However, out study had the limitation that pregnancy outcome was not assessed because not only the embryos of the single dominant follicle were included in the embryo transfer. Thus, if two or more embryos were transferred, we could not be sure whether the pregnancy had originated from the embryo analyzed in this study. We did not transfer the poor quality embryos that were included in this study. This is also related to ethical concerns. In addition, it is difficult to generalize to natural pregnancy because we controlled the folliculogenesis by gonadotropin in an IVF setting. These are the limitations of this study. Future research on the pregnancy outcomes of oocytes derived from a single dominant follicle is needed.
In conclusion, the present study demonstrates that intrafollicular adiponectin concentrations were significantly associated with the normal fertilization rate. Low levels of leptin and high levels of adiponectin, as well as a good intrafollicular adipocytokine environment seem to modulate the function of the ovarian follicles and improve the quality of oocytes and embryos. However, the utility of follicular levels of adipocytokines has not been definitively established as a prognostic marker of IVF/ICSI cycles.
Notes
References
1. Fedorcsak P, Dale PO, Storeng R, Ertzeid G, Bjercke S, Oldereid N, et al. Impact of overweight and underweight on assisted reproduction treatment. Hum Reprod 2004;19:2523-2528.PMID: 15319380.


2. Metwally M, Ong KJ, Ledger WL, Li TC. Does high body mass index increase the risk of miscarriage after spontaneous and assisted conception? A meta-analysis of the evidence. Fertil Steril 2008;90:714-726.PMID: 18068166.


3. Maheshwari A, Stofberg L, Bhattacharya S. Effect of overweight and obesity on assisted reproductive technology: a systematic review. Hum Reprod Update 2007;13:433-444.PMID: 17584821.


4. Lintsen AM, Pasker-de Jong PC, de Boer EJ, Burger CW, Jansen CA, Braat DD, et al. Effects of subfertility cause, smoking and body weight on the success rate of IVF. Hum Reprod 2005;20:1867-1875.PMID: 15817580.


5. Wang JX, Davies M, Norman RJ. Body mass and probability of pregnancy during assisted reproduction treatment: retrospective study. BMJ 2000;321:1320-1321.PMID: 11090515.



6. Fedorcsak P, Storeng R, Dale PO, Tanbo T, Abyholm T. Obesity is a risk factor for early pregnancy loss after IVF or ICSI. Acta Obstet Gynecol Scand 2000;79:43-48.PMID: 10646815.


7. ESHRE Task Force on Ethics and Law, including. Dondorp W, de Wert G, Pennings G, Shenfield F, Devroey P, et al. Lifestyle-related factors and access to medically assisted reproduction. Hum Reprod 2010;25:578-583.PMID: 20085914.


8. van der Steeg JW, Steures P, Eijkemans MJ, Habbema JD, Hompes PG, Michgelsen HW, et al. Predictive value of pregnancy history in subfertile couples: results from a nationwide cohort study in the Netherlands. Fertil Steril 2008;90:521-527.PMID: 17980877.


9. Brewer CJ, Balen AH. The adverse effects of obesity on conception and implantation. Reproduction 2010;140:347-364.PMID: 20395425.


10. van der Steeg JW, Steures P, Eijkemans MJ, Habbema JD, Hompes PG, Burggraaff JM, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod 2008;23:324-328.PMID: 18077317.


11. Jensen TK, Scheike T, Keiding N, Schaumburg I, Grandjean P. Fecundability in relation to body mass and menstrual cycle patterns. Epidemiology 1999;10:422-428.PMID: 10401878.


12. Robker RL, Akison LK, Bennett BD, Thrupp PN, Chura LR, Russell DL, et al. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J Clin Endocrinol Metab 2009;94:1533-1540.PMID: 19223519.


13. Chudek J, Adamczak M, Nieszporek T, Wiecek A. The adipose tissue as an endocrine organ: a nephrologists’ perspective. Contrib Nephrol 2006;151:70-90.PMID: 16929134.


14. Wozniak SE, Gee LL, Wachtel MS, Frezza EE. Adipose tissue: the new endocrine organ? A review article. Dig Dis Sci 2009;54:1847-1856.PMID: 19052866.


15. Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol 2010;316:129-139.PMID: 19723556.


16. Li L, Ferin M, Sauer MV, Lobo RA. Ovarian adipocytokines are associated with early in vitro human embryo development independent of the action of ovarian insulin. J Assist Reprod Genet 2012;29:1397-1404.PMID: 23054357.



17. Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, et al. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab 1996;81:3424-3427.PMID: 8784109.


18. Meilleur KG, Doumatey A, Huang H, Charles B, Chen G, Zhou J, et al. Circulating adiponectin is associated with obesity and serum lipids in West Africans. J Clin Endocrinol Metab 2010;95:3517-3521.PMID: 20382687.



19. Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 1996;334:292-295.PMID: 8532024.


20. Anifandis G, Koutselini E, Stefanidis I, Liakopoulos V, Leivaditis C, Mantzavinos T, et al. Serum and follicular fluid leptin levels are correlated with human embryo quality. Reproduction 2005;130:917-921.PMID: 16322551.


21. Barroso G, Barrionuevo M, Rao P, Graham L, Danforth D, Huey S, et al. Vascular endothelial growth factor, nitric oxide, and leptin follicular fluid levels correlate negatively with embryo quality in IVF patients. Fertil Steril 1999;72:1024-1026.PMID: 10593375.


22. Welt CK, Schneyer AL, Heist K, Mantzoros CS. Leptin and soluble leptin receptor in follicular fluid. J Assist Reprod Genet 2003;20:495-501.PMID: 15035548.



23. Asimakopoulos B, Koster F, Felberbaum R, Tripsiannis G, Caglar GS, Nikolettos N, et al. Intrafollicular and circulating concentrations of leptin do not predict the outcome in IVF-ICSI cycles. Reprod Sci 2009;16:113-119.PMID: 19144893.


24. Chang HJ, Lee JR, Jee BC, Suh CS, Kim SH. Cessation of gonadotropin-releasing hormone antagonist on triggering day: an alternative method for flexible multiple-dose protocol. J Korean Med Sci 2009;24:262-268.PMID: 19399268.



25. Brannian JD, Hansen KA. Leptin and ovarian folliculogenesis: implications for ovulation induction and ART outcomes. Semin Reprod Med 2002;20:103-112.PMID: 12087495.


26. Adamczak M, Wiecek A. The adipose tissue as an endocrine organ. Semin Nephrol 2013;33:2-13.PMID: 23374889.


27. Ryan NK, Woodhouse CM, Van der Hoek KH, Gilchrist RB, Armstrong DT, Norman RJ. Expression of leptin and its receptor in the murine ovary: possible role in the regulation of oocyte maturation. Biol Reprod 2002;66:1548-1554.PMID: 11967222.


28. Craig J, Zhu H, Dyce PW, Petrik J, Li J. Leptin enhances oocyte nuclear and cytoplasmic maturation via the mitogen-activated protein kinase pathway. Endocrinology 2004;145:5355-5363.PMID: 15284194.


29. Boelhauve M, Sinowatz F, Wolf E, Paula-Lopes FF. Maturation of bovine oocytes in the presence of leptin improves development and reduces apoptosis of in vitro-produced blastocysts. Biol Reprod 2005;73:737-744.PMID: 15958729.


30. Ye Y, Kawamura K, Sasaki M, Kawamura N, Groenen P, Sollewijn Gelpke MD, et al. Leptin and ObRa/MEK signalling in mouse oocyte maturation and preimplantation embryo development. Reprod Biomed Online 2009;19:181-190.PMID: 19712552.


31. Joo JK, Joo BS, Kim SC, Choi JR, Park SH, Lee KS. Role of leptin in improvement of oocyte quality by regulation of ovarian angiogenesis. Anim Reprod Sci 2010;119:329-334.PMID: 20197222.


32. Tsai EM, Yang CH, Chen SC, Liu YH, Chen HS, Hsu SC, et al. Leptin affects pregnancy outcome of in vitro fertilization and steroidogenesis of human granulosa cells. J Assist Reprod Genet 2002;19:169-176.PMID: 12036084.



33. Asimakopoulos B, Nikolettos N, Papachristou DN, Simopoulou M, Al-Hasani S, Diedrich K. Follicular fluid levels of vascular endothelial growth factor and leptin are associated with pregnancy outcome of normal women participating in intracytoplasmic sperm injection cycles. Physiol Res 2005;54:263-270.PMID: 15588162.


34. Chabrolle C, Tosca L, Dupont J. Regulation of adiponectin and its receptors in rat ovary by human chorionic gonadotrophin treatment and potential involvement of adiponectin in granulosa cell steroidogenesis. Reproduction 2007;133:719-731.PMID: 17504916.


35. Kim ST, Marquard K, Stephens S, Louden E, Allsworth J, Moley KH. Adiponectin and adiponectin receptors in the mouse preimplantation embryo and uterus. Hum Reprod 2011;26:82-95.PMID: 21106494.


36. Cikos S, Burkus J, Bukovska A, Fabian D, Rehak P, Koppel J. Expression of adiponectin receptors and effects of adiponectin isoforms in mouse preimplantation embryos. Hum Reprod 2010;25:2247-2255.PMID: 20663797.


37. Chappaz E, Albornoz MS, Campos D, Che L, Palin MF, Murphy BD, et al. Adiponectin enhances in vitro development of swine embryos. Domest Anim Endocrinol 2008;35:198-207.PMID: 18638663.


38. Richards JS, Liu Z, Kawai T, Tabata K, Watanabe H, Suresh D, et al. Adiponectin and its receptors modulate granulosa cell and cumulus cell functions, fertility, and early embryo development in the mouse and human. Fertil Steril 2012;98:471-479.e1.PMID: 22633650.



39. Takemura Y, Osuga Y, Yamauchi T, Kobayashi M, Harada M, Hirata T, et al. Expression of adiponectin receptors and its possible implication in the human endometrium. Endocrinology 2006;147:3203-3210.PMID: 16601138.