 |
 |
- Search
| Clin Exp Reprod Med > Volume 47(3); 2020 > Article |
|
Abstract
Objective
Oxidative stress has been suggested as a possible mechanism for the adverse effects of heavy metal toxicity on male reproduction. Cichorium intybus L. is used in Iranian folk medicine as a hepatoprotective agent as well as for its supposed fertility-enhancing properties. The present study was performed to investigate whether the ethanolic extract of C. intybus leaves could protect male rats against lead-induced testicular oxidative stress.
Methods
In this experimental study, adult Wistar rats were treated with 0.1% lead acetate in drinking water alone or with 50, 100, or 200 mg/ kg body weight of C. intybus extract via gavage once daily for 70 days. The weight of their reproductive organs, levels of serum hormones, histometric parameters of the seminiferous tubules, epidydimal sperm quality, and oxidative stress status were evaluated.
Results
The testis weight, seminiferous tubule diameter, epididymal sperm count, serum testosterone level, and testicular levels of superoxide dismutase and glutathione peroxidase were significantly reduced (p<0.05) in the lead-treated rats. Moreover, significantly (p<0.05) higher levels of malondialdehyde were observed in the lead-exposed group compared to the control. However, the co-administration of C. intybus ethanolic extract in lead-treated rats was associated with a significant improvement in reproductive parameters.
Over recent decades, concern has been increasing regarding the declining human sperm count. Environmental, occupational, lifestyle, and dietary factors may affect reproductive health and contribute to decreases in semen quality [1,2]. Heavy metals adversely impact male reproductive health [3], even at relatively low levels of exposure [4], via either the disruption of the hypothalamic-pituitary axis or direct effects on spermatogenesis leading to reduced semen quality [5].
Lead is an environmental pollutant that can induce abnormalities in male reproductive function [6]. Significant negative correlations have been reported between semen quality and lead concentrations in the semen or blood [7]. Telisman et al. [4] found that blood lead levels in a group of Croatian men was positively associated with the percentage of pathological sperm. Lead levels in the sperm of Mexican men residing in urban areas were also demonstrated to show significant negative associations with sperm concentration, motility, viability, and normal morphology [8].
Oxidative stress, the major mechanism of lead toxicity, has been reported to occur as a result of reduced activity of antioxidant enzymes [9] and increased production of reactive oxygen species (ROS) [10], leading to lipid peroxidation and protein and nucleic acid oxidation [11]. Because the key constituents of the sperm cell membraneŌĆö polyunsaturated fatty acids and phospholipidsŌĆöare highly susceptible to oxidative damage, high concentrations of free radicals such as the superoxide anion, hydrogen peroxide, and nitric oxide directly damage the cells. This damage has been proposed as a possible etiology of idiopathic male infertility [12]. Accordingly, it has been suggested that antioxidants may protect body systems against various deleterious effects of lead [13].
In this regard, some believe that the adverse effects of toxic chemicals such as heavy metals may be mitigated by herbal remedies. Cichorium intybus L. is an herbaceous plant of the Asteraceae family that grows across much of Asia, Africa, and Europe. In the Ayurvedic and Unani systems of traditional medicine, C. intybus has been used to cure various ailments and has also been found to have wide-ranging applications in the food industry [14]. In Iranian folk medicine, C. intybus is known as kasni and has been used traditionally for its hepatoprotective, blood purifying, and male fertility-promoting properties [15-17]. Our previous study showed that C. intybus leaf extract improved reproductive parameters in male rats [18]. The whole extract of C. intybus has been reported to have anti-diabetic, antibacterial, immunostimulatory, and cardioprotective properties [19]. Phytochemical analyses have shown that C. intybus extract contains polyphenols such as chlorogenic acid, fructooligosaccharides, inulin, and caffeic acid derivatives [20]. Therefore, the present study was designed to evaluate the ability of C. intybus extract to protect against lead-induced oxidative stress and reproductive toxicity in adult male Wistar rats.
The leaves of C. intybus L. were collected from areas near Behbahan in Khouzestan, Iran, and authentication was conducted at the Botanical Systematic Laboratory, Department of Biology at Shahid Chamran University of Ahvaz in Iran. The dried C. intybus leaves (200 g) were ground to a fine powder and extracted using maceration with 96% ethanol (Merck, Darmstadt, Germany). The extract was filtered, and the solvent was evaporated using a rotary evaporator at 50┬░C. The extract was stored at 4┬░C until the time of use.
Fifty healthy 8-week-old male Wistar rats (body weight, 180ŌĆō200 g) were housed under standard conditions with regard to temperature (23┬░C ┬▒ 2┬░C) and light/dark cycle (12 hr/12 hr) and were given free access to standard food and water. The study protocol was approved by the Animal Ethics Committee of the Department of Biology at Shahid Chamran University of Ahvaz in Ahvaz, Iran. All experimental procedures were performed in accordance with National Institutes of Health guidelines for the care and use of laboratory animals (National Institutes of Health Publications No. 8023, revised 1978). The animals were randomly divided into five groups (n=10). The rats in the control group were gavaged with 1 mL of distilled water once daily. The rats of the second group were allowed to drink distilled water containing 0.1% lead acetate ad libitum and were given 1 mL of distilled water through gavage once daily. The rats in the remaining three groups were allowed to drink distilled water containing 0.1% lead acetate ad libitum and were treated, respectively, with 50, 100, and 200 mg/kg body weight of C. intybus extract via gavage once daily. The dose of lead acetate was chosen based on previously published studies and continued for 70 Consecutive days [21,22]. After 70 days, the animals were weighed and sacrificed under light ether anesthesia. Blood samples were collected in laboratory tubes via cardiac puncture and centrifuged at 3,000 rpm for 15 minutes, after which the reproductive organs were removed and weighed. The serum lead levels were estimated using the graphite furnace atomic absorption spectrometry method in the K Kimiay-e-Nab imiay-e-Nab analysis laboratory in Karaj, Iran.
The right testes were excised, cleaned, and fixed in Bouin solution. Then, 5-╬╝m-thick paraffin sections were stained with hematoxylin and eosin for evaluation by light microscopy (Olympus, Tokyo, Japan). To measure the seminiferous tubule diameter, 2 perpendicular diameters of each cross-section were measured in 90 randomly chosen, nearly round cross-sections of the seminiferous tubules in each rat at a magnification of ├Ś 40.
The cauda epididymis was excised in a petri dish containing 1.0 mL of phosphate buffer (0.1 M, pH 7.4). The dishes were gently swirled for 10 minutes at 37┬░C to allow dispersion of the sperm cells in the solution. A 10-╬╝L aliquot of epididymal sperm suspension was placed on a hemocytometer and allowed to stand for 5 minutes; then, the sperm concentration was assessed and expressed in millions/mL of suspension. To gauge sperm motility, 10 ╬╝L of suspension was evaluated using a hemocytometer (Paul Marienfeld, Lauda-Konigshofen, Germany), and the percentages of motile and non-motile sperm were counted. To analyze sperm morphology, 40 ╬╝L of sperm suspension was mixed with 10 ╬╝L of 1% eosin Y for 45ŌĆō60 minutes at room temperature, and the percent of morphologically abnormal sperm was recorded.
The left testes were homogenized in sodium phosphate buffer (0.1 M) at 4┬░C and centrifuged at 10,000 rpm for 15ŌĆō20 minutes at 4┬░C, and the supernatants were stored at ŌłÆ20┬░C until analysis. The activity levels of glutathione peroxidase (GPx) and superoxide dismutase (SOD) in the testicular homogenate were estimated using assay kits according to the manufacturerŌĆÖs instructions (Randox Laboratories, Crumlin, UK). The testicular levels of malondialdehyde (MDA) were determined using the thiobarbituric acid reaction and measured spectrophotometrically at 532 nm [23].
Serum levels of testosterone, luteinizing hormone (LH), and folliclestimulating hormone (FSH) were estimated using enzyme-linked immunosorbent assays (ELISA), using a commercial kit (AccuBind ELISA kit; Monobind Inc., Lake Forest, CA, USA) according to the manufacturerŌĆÖs instructions.
Significant (p<0.05) decreases were observed in the weights of the testis, epididymis, seminal vesicle, and ventral prostate in lead acetate-treated rats compared with the control group (Table 1). Dose-dependent increases (p<0.05) were observed in the weights of the testis, epididymis, seminal vesicle, and ventral prostate in rats co-administered C. intybus extract relative to the lead acetate-treated rats (Table 1).
In the rats exposed to lead acetate, the seminiferous tubule diameter was significantly lower (p< 0.05) than in the control rats (Figure 1). The seminiferous tubule diameter was significantly higher (p< 0.05) in a dose-response manner in the lead acetate-treated rats co-administered C. intybus extract than in the rats administered lead acetate alone (Table 1).
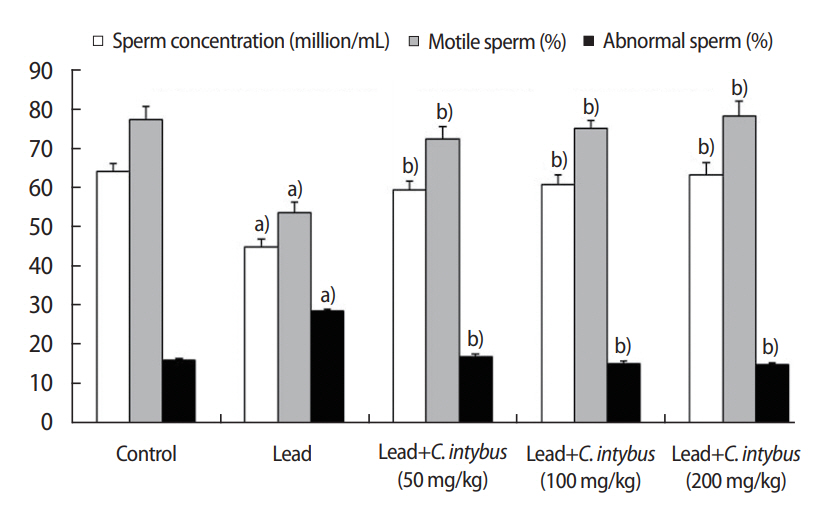
In the lead acetate-treated rats, relative to the control group, significant (p< 0.05) decreases were observed in the sperm concentration and the percent of motile sperm. A higher percentage of sperm with abnormal morphology (p< 0.05) was also observed in the lead acetate-treated rats (Figure 2). The coadministration of C. intybus extract significantly improved (p<0.05) sperm parameters in a dose-response manner compared with the lead acetate-treated rats (Figure 3).
In the rats treated with lead acetate, the testicular levels of MDA were significantly higher (p< 0.05) than in the control group. Moreover, the activity levels of SOD and GPx were significantly lower (p< 0.05) in the testes of the rats exposed to lead acetate (Table 2). In a dose-dependent manner, MDA levels were shown to decrease and the levels of activity of SOD and GPx were shown to increase significantly (p< 0.05) in the rats coadministered C. intybus extract (Table 2).
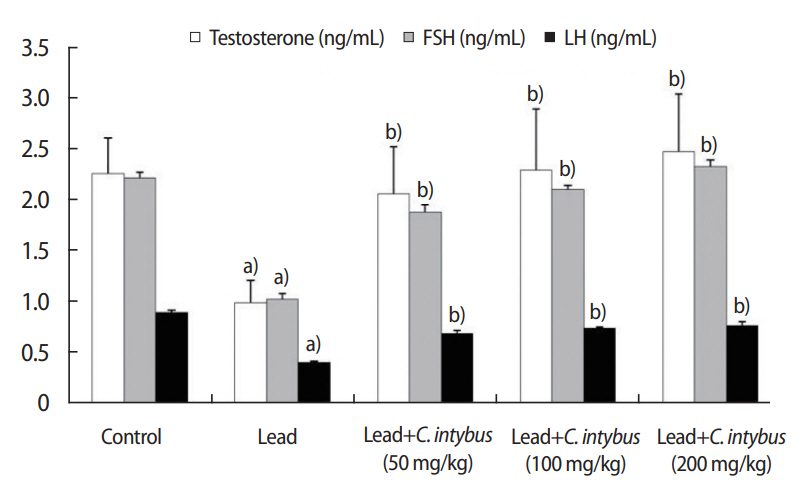
Compared with the control group, significant (p< 0.05) declines were seen in serum testosterone, FSH, and LH levels in the lead acetate-treated rats (Figure 4). In the rats co-administered C. intybus extract, significantly (p< 0.05) higher levels of testosterone, FSH, and LH were observed compared with the lead acetate-treated group, and these results were dose-dependent (Figure 4).
Traditional herbal remedies are a complementary therapy used to enhance fertility and to improve semen parameters in infertile men. The aim of the present study was to evaluate the potential protective effects of C. intybus leaf extract on lead-induced oxidative reproductive toxicity in male Wistar rats. C. intybus is known in different areas of the world for its numerous properties in traditional medicine and is used in Iranian folk medicine for its supposed fertility-enhancing properties. Given that exposure to heavy metals can trigger lipid peroxidation and cause oxidative stress damage, antioxidants may be an effective defense mechanism against lead-induced toxicity. In this study, the effects of co-administration of C. intybus leaf extract on reproductive and oxidative status parameters were evaluated in male rats exposed to low levels of lead acetate. The results showed that coadministration of C. intybus mitigates testicular toxicity and improves male reproductive function in lead-treated rats.
Our findings demonstrated that exposure to lead acetate adversely affects reproductive parameters in adult male rats. The toxic effects of lead on male reproductive function may be direct (via affecting spermatogenesis and sperm function) or indirect (by disturbing the hypothalamic-pituitary-testicular axis) [22,24]. Weighing reproductive organs is widely considered to be a valuable screening tool [25]. In the present study, the weight of the testis was significantly decreased in lead-treated rats, a finding that aligned with earlier reports [26-28]. Crucially, the disruption of spermatogenesis has been shown to reduce the mass of differentiated spermatogenic cells and lead to a decrease in the testis weight [29]. In addition, the weights of the testes and male accessory reproductive glands are known to be positively correlated with testosterone levels, meaning that the adequate bioavailability of testosterone is essential for the structural and functional integrity of reproductive organs [30]. Therefore, a reduction in serum testosterone levels due to a decrease in androgen biosynthesis led to significant decreases in the weights of the testis, prostate, and seminal vesicle in the lead-treated rats.
However, the co-administration of C. intybus leaf extract resulted in dose-related increases in epididymal sperm count in the lead-treated rats. Epididymal sperm count is considered to be one of the most sensitive tests used to evaluate testicular function, sperm production, and male fertility [31]. The decreases in serum testosterone levels explain the reduction in sperm count even in light of the finding of epidydimal weight loss in lead-treated rats [32]. Thus, lead toxicity could cause a reduction in sperm count and an increase in the percentage of abnormal spermatozoa, resulting in reduced fertility [33]. The increases in sperm count and the percent of normal morphological sperm in lead-treated rats therefore indicate that treatment with C. intybus improves and enhances the fertilizing capacity of semen. In addition, increased levels of MDA and decreased activity of SOD and GPx in the testes of adult rats show that exposure to lead results in testicular oxidative stress. Our findings are consistent with the results of previous reports [26,34]. The excessive generation of ROS overwhelms the bodyŌĆÖs antioxidant defense and leads to oxidative stress, which has been reported to be a major mechanism underlying lead toxicity [35]. The binding of lead to sulfhydryl groups or metal cofactors of antioxidant enzymes such as SOD and GPx reduces the activity of these enzymes [36]. Higher levels of ROS have been found to be associated with impaired sperm motility, decreased concentration, and altered morphology [37]. The generation of free radicals results in lipid peroxidation in the plasma membrane, which is known to play a significant role in the etiology of sperm dysfunction [38].
This study showed that C. intybus leaf extract improves testicular oxidative status by decreasing the levels of lipid peroxidation and increasing the activity of SOD and GPx in the testes. In vitro studies have indicated that the constituents of C. intybus, especially from the red varieties of the plant, may possess anti-free radical activity [39]. Previous studies have shown that C. intybus extract could protect against oxidative stress and hepatotoxicity induced by paracetamol or carbon tetrachloride [40-42]. Phytochemical analyses have shown that the phenolic compounds that are present in large amounts in C. intybus alcoholic extract are important contributors to its antioxidant properties [14,43]. The protective properties of phenolic compounds against cellular oxidative damage stem from their hydrogen donating ability [44]. Blokhina et al. [45] showed that phenolic compounds hinder the diffusion of free radicals, restrict the peroxidative reaction, and reduce membrane fluidity and thereby stabilize cell membranes [45]. However, this is not only true of phenolic compounds; rather, the sugars of chicory preparations, especially sucrose and fructans, have also been proposed to act as radical scavengers in plant cells [46]. Consequently, the present study showed that the co-administration of C. intybus extract attenuated lead-induced testicular oxidative stress and toxicity by reducing lipid peroxidation and increasing the activity of antioxidant enzymes. This co-administration thereby improved reproductive efficiency in lead-treated adult male rats. However, whether C. intybus extract can reverse the reproductive effects of lead-induced toxicity requires further study. Our findings support the beneficial effects of C. intybus on male reproductive health and its traditional use for the treatment of male infertility. However, the study was limited by the lack of available information regarding the safety of C. intybus extract. Further investigations are needed to evaluate the toxicity of C. intybus extract and the therapeutic efficacy of its bioactive constituents.
Figure┬Ā1.
Histological sections of the testis (H&E, ├Ś 40) in adult Wistar rats in (A) control rats, (B) lead-treated rats, and (C) lead-treated rats coadministered Cichorium intybus extract. In the lead-treated rats, the degeneration of the seminiferous tubule epithelium, decreased spermatogenesis, decreased germinal epithelium height (red arrows), and the increased size of the tubular lumen were observed. L, lumen.

Figure┬Ā2.
Spermatozoa in adult male rats with (A) normal and (B, C) abnormal morphology (H&E, ├Ś40). Red arrows: (B) bent midpiece; (C) bent tail.

Figure┬Ā3.
Sperm parameters in different groups. Values are presented as mean ┬▒ standard deviation. C, Cichorium. a)Significant difference (p< 0.05) with respect to control rats; b)Significant difference (p< 0.05) with respect to rats treated with lead acetate.
Figure┬Ā4.
Serum testosterone, follicle-stimulating hormone (FSH) levels, and luteinizing hormone (LH) in the different groups. Values are presented as mean ┬▒ standard deviation. C, Cichorium. a)Significant difference (p<0.05) with respect to control rats; b)Significant difference (p<0.05) with respect to rats treated with lead acetate.
Table┬Ā1.
Body and reproductive organs weight and seminiferous tubules diameter in different groups
| Variable | Control | Pb | Pb+C. intybus (50 mg/kg) | Pb+C. intybus (100 mg/kg) | Pb+C.intybus (200 mg/kg) |
|---|---|---|---|---|---|
| Body (g) | 300.5 ┬▒ 2.8 | 282.2 ┬▒ 5.3a) | 310.3 ┬▒ 3.4b) | 308.2 ┬▒ 2.3b) | 307.2 ┬▒ 2.1b) |
| Testis (mg) | 1,322.2 ┬▒ 16.0 | 1,004.1 ┬▒ 15.1a) | 1242.5 ┬▒ 18.0b) | 1314.3 ┬▒ 16.4b) | 1329.1 ┬▒ 14.6b) |
| STD (┬Ąm) | 262.6 ┬▒ 2.5 | 241.7 ┬▒ 3.2a) | 255.3 ┬▒ 2.8b) | 258.1 ┬▒ 2.1b) | 262.2 ┬▒ 2.6b) |
| Epididymis (mg) | 451.3 ┬▒ 7.7 | 335.4 ┬▒ 6.8a) | 410.2 ┬▒ 7.3b) | 435.1 ┬▒ 6.4b) | 450.8 ┬▒ 6.7b) |
| Seminal vesicle (mg) | 432.2 ┬▒ 9.7 | 334.7 ┬▒ 10.1a) | 419.6 ┬▒ 6.4b) | 422.2 ┬▒ 6.5b) | 439.4 ┬▒ 5.2b) |
| Ventral prostate (mg) | 194.8 ┬▒ 4.2 | 146.6 ┬▒ 2.3a) | 171.7 ┬▒ 5.3b) | 177.6 ┬▒ 4.7b) | 189.6 ┬▒ 3.2b) |
Table┬Ā2.
Lipid peroxidation and antioxidant enzymes levels in different groups
| Variable | Control | Pb | Pb+C. intybus (50 mg/kg) | Pb+C. intybus (100 mg/kg) | Pb+C. intybus (200 mg/kg) |
|---|---|---|---|---|---|
| MDA (nmol/mg protein) | 7.55 ┬▒ 0.55 | 12.23 ┬▒ 0.72a) | 8.21 ┬▒ 0.41b) | 7.83 ┬▒ 0.44b) | 7.24 ┬▒ 0.53b) |
| GPx (U/mg protein) | 13.21 ┬▒ 0.49 | 4.84 ┬▒ 0.39a) | 10.48 ┬▒ 0.54b) | 12.26 ┬▒ 0.6b) | 13.67 ┬▒ 0.61b) |
| SOD (U/mg protein) | 1.24 ┬▒ 0.15 | 0.56 ┬▒ 0.15a) | 1.10 ┬▒ 0.15b) | 1.22 ┬▒ 0.14b) | 1.27 ┬▒ 0.14b) |
References
1. Jensen TK, Bonde JP, Joffe M. The influence of occupational exposure on male reproductive function. Occup Med (Lond) 2006;56:544-53.



2. Basnet P, Hansen SA, Olaussen IK, Hentemann MA, Acharya G. Changes in the semen quality among 5739 men seeking infertility treatment in Northern Norway over past 20 years (1993- 2012). J Reprod Biotechnol Fertil 2016;5:1-7.

3. Meeker JD, Rossano MG, Protas B, Diamond MP, Puscheck E, Daly D, et al. Cadmium, lead, and other metals in relation to semen quality: human evidence for molybdenum as a male reproductive toxicant. Environ Health Perspect 2008;116:1473-9.



4. Telisman S, Colak B, Pizent A, Jurasovic J, Cvitkovic P. Reproductive toxicity of low-level lead exposure in men. Environ Res 2007;105:256-66.


5. Wyrobek AJ, Schrader SM, Perreault SD, Fenster L, Huszar G, Katz DF, et al. Assessment of reproductive disorders and birth defects in communities near hazardous chemical sites. III. Guidelines for field studies of male reproductive disorders. Reprod Toxicol 1997;11(2-3): 243-59.
6. Kumar S. Occupational and environmental exposure to lead and reproductive health impairment: an overview. Indian J Occup Environ Med 2018;22:128-37.



7. Eibensteiner L, Del Carpio Sanz A, Frumkin H, Gonzales C, Gonzales GF. Lead exposure and semen quality among traffic police in Arequipa, Peru. Int J Occup Environ Health 2005;11:161-6.


8. Hernandez-Ochoa I, Garcia-Vargas G, Lopez-Carrillo L, Rubio-Andrade M, Moran-Mart├Łnez J, Cebrian ME, et al. Low lead environmental exposure alters semen quality and sperm chromatin condensation in northern Mexico. Reprod Toxicol 2005;20:221-8.


9. Bolin CM, Basha R, Cox D, Zawia NH, Maloney B, Lahiri DK, et al. Exposure to lead and the developmental origin of oxidative DNA damage in the aging brain. FASEB J 2006;20:788-90.


10. Marchlewicz M, Michalska T, Wiszniewska B. Detection of leadinduced oxidative stress in the rat epididymis by chemiluminescence. Chemosphere 2004;57:1553-62.


11. Gurer H, Ercal N. Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic Biol Med 2000;29:927-45.


12. de Lamirande E, Jiang H, Zini A, Kodama H, Gagnon C. Reactive oxygen species and sperm physiology. Rev Reprod 1997;2:48-54.


13. Shalan MG, Mostafa MS, Hassouna MM, El-Nabi SE, El-Refaie A. Amelioration of lead toxicity on rat liver with Vitamin C and silymarin supplements. Toxicology 2005;206:1-15.


14. Ilaiyaraja N, Khanum F. Evaluation of antioxidant and toxicological properties of chicory leaves. IJPBA 2010;1:155-63.
15. Mirhaydar H. Plants sciences: application of plants in prophylaxisand treatment of diseases. Tehran, Daftar Nashr Farhang Islami. 1993.
16. Zargari A. Medicinal plants. Tehran: Tehran University Publication; 1996.
17. Behnam Rasouli M, Hosseinzade H, Ali Akbarpour A. The effects of Soxhlet aqueous extract of Chicory leaves on the blood pH and cations level & the sex of newborns in rat. Daneshvar Med 2000;27:57-64.
18. Dorostghoal M, Seyyednejad SM, Nejad MN. Beneficial effects of Cichorium intybus L. extract on oxidative status and reproductive parameters in male Wistar rats: An experimental study. Int J Reprod Biomed (Yazd) 2019;17:425-34.

19. Das S, Vasudeva N, Sharma S. Cichorium intybus: A concise report on its ethnomedicinal, botanical, and phytopharmacological aspects. Drug Dev Ther 2016;7:1-12.

20. Kim JH, Mun YJ, Woo WH, Jeon KS, An NH, Park JS. Effects of the ethanol extract of Cichorium intybus on the immunotoxicity by ethanol in mice. Int Immunopharmacol 2002;2:733-44.


21. Hayes W. Principle and methods of toxicology. New York: Raven Press; 1989.
22. El-Sayed YS, El-Neweshy MS. Impact of lead toxicity on male rat reproduction at hormonal and histopathological levels. Toxicol Environ Chem 2010;4:765-74.

23. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351-8.


24. Rubio J, Riqueros MI, Gasco M, Yucra S, Miranda S, Gonzales GF. Lepidium meyenii (Maca) reversed the lead acetate induced- -damage on reproductive function in male rats. Food Chem Toxicol 2006;44:1114-22.


25. Michael B, Yano B, Sellers RS, Perry R, Morton D, Roome N, et al. Evaluation of organ weights for rodent and non-rodent toxicity studies: a review of regulatory guidelines and a survey of current practices. Toxicol Pathol 2007;35:742-50.


26. Sainath SB, Meena R, Supriya Ch, Reddy KP, Reddy PS. Protective role of Centella asiatica on lead-induced oxidative stress and suppressed reproductive health in male rats. Environ Toxicol Pharmacol 2011;32:146-54.


27. Hamadouche NA, Sadi N, Kharoubi O, Slimani M, Aoues A. The protective effect of vitamin E against genotoxicity of lead acetate intraperitoneal administration in male rat. Arch Biol Sci 2013;65:1435-45.

28. Anjum MR, Madhu P, Reddy KP, Reddy PS. The protective effects of zinc in lead-induced testicular and epididymal toxicity in Wistar rats. Toxicol Ind Health 2017;33:265-76.


29. Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev 2004;25:747-806.



30. Yoshida M, Kitani T, Takenaka A, Kudoh K, Katsuda SI, Taya K, et al. Lack of effects of oxolinic acid on spermatogenesis in young adult and aged Wistar rats. Food Chem Toxicol 2002;40:1815-25.


31. Chandra AK, Ghosh R, Chatterjee A, Sarkar M. Effects of vanadate on male rat reproductive tract histology, oxidative stress markers and androgenic enzyme activities. J Inorg Biochem 2007;101:944-56.


32. Uzunhisarcikli M, Kalender Y, Dirican K, Kalender S, Ogutcu A, Buyukkomurcu F. Acute, subacute and subchronic administration of methyl parathion-induced testicular damage in male rats and protective role of vitamins C and E. Pestic Biochem Physiol 2007;87:115-22.

33. Raji Y, Udoh US, Mewoyeka OO, Ononye FC, Bolarinwa AF. Implication of reproductive endocrine malfunction in male antifertility efficacy of Azadirachta indica extract in rats. Afr J Med Sci 2003;32:159-65.
34. Dobrakowski M, Pawlas N, Kasperczyk A, Kozlowska A, Olewinska E, Machon-Grecka A, et al. Oxidative DNA damage and oxidative stress in lead-exposed workers. Hum Exp Toxicol 2017;36:744-54.


35. Martinez-Haro M, Green AJ, Mateo R. Effects of lead exposure on oxidative stress biomarkers and plasma biochemistry in waterbirds in the field. Environ Res 2011;111:530-8.


36. Patrick L. Lead toxicity part II: the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern Med Rev 2006;11:114-27.

37. Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health 2014;32:1-17.



38. Aitken RJ, Smith TB, Jobling MS, Baker MA, De Iuliis GN. Oxidative stress and male reproductive health. Asian J Androl 2014;16:31-8.


39. Jurgonbski A, Milala J, Jusbkiewicz J, Zdunbczyk Z, Boguslaw Krol. Composition of chicory root, peel, seed and leaf ethanol extracts and biological properties of their non-inulin fractions. Food Technol Biotechnol 2011;49:40-7.
40. Jamshidzadeh A, Khoshnood MJ, Dehghani Z, Niknahad H. Hepatoprotective activity of Cichorium intybus L. leaves extract against carbon tetrachloride induced toxicity. Iran J Pharm Res 2006;5:41-6.
41. Gadgoli C, Mishra SH. Antihepatotoxic activity of Cichorium intybus. J Ethnopharmacol 1997;58:131-4.


42. Ahmed B, Al-Howiriny TA, Siddiqui AB. Antihepatotoxic activity of seeds of Cichorium intybus. J Ethnopharmacol 2003;87:237-40.


43. Rice-Evans C, Miller N, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci 1997;2:152-9.

44. Sakihama Y, Mano J, Sano S, Asada K, Yamasaki H. Reduction of phenoxyl radicals mediated by monodehydroascorbate reductase. Biochem Biophys Res Commun 2000;279:949-54.


- TOOLS







