 |
 |
- Search
| Clin Exp Reprod Med > Volume 47(3); 2020 > Article |
|
Abstract
Objective
The effectiveness of Staphylococcus protein A (SPA) in improving the penetration ability of sperm and reducing antisperm antibody (ASA) titers in immunologically infertile males was evaluated.
Methods
Seminal fluid samples were obtained from 15 infertile men, and ASA titers were assessed with the latex agglutination test. Identification of immunoglobulin (Ig) classes and characterization of the antigens involved in the immune response were performed using indirect immunofluorescence. Local ASAs typically present as a mixture of IgG and IgA classes. The capillary tube penetration method was used to assess the capability of spermatozoa to penetrate the cervical mucus (CM).
Results
ASAs associated with the neck region of sperm showed a significantly lower migration distance in the CM of infertile females than ASAs associated with the head or tail segments. ASA-positive seminal fluid exhibited significant increases in the mean migration distance (2.6 ┬▒ 1.4 cm vs. 1.54 ┬▒ 1.1 cm, respectively; p< 0.001) and sperm concentration (174 ┬▒ 121.0 ├Ś 10┬│/mL vs. 101 ┬▒ 93.7 ├Ś 10┬│/mL, respectively; p= 0.033) after treatment with SPA compared to pre-treated samples. A significant reduction (p< 0.01) in the recorded ASA titer was detected.
Infertility is a global health issue that affects approximately 8%ŌĆō10% of couples [1]. Antisperm antibodies (ASAs) are an immunological marker involved in male and female infertility [2]. Fewer than 10% of males with infertility of all etiologies test positive for ASA [3]. ASAs act by blocking sperm movement, capacitation, fertilization, and by inhibiting embryonic implantation [4]. These antibodies are developed against specific sperm antigens, such as nuclear autoantigenic sperm protein, fertilization antigen-1, and acrosin antigens that inhibit the binding of sperm with the zona pellucida through protease activity [5].
ASAs inhibit sperm progression in the female genital tract, as is often demonstrated by poor postcoital test (PCT) and cervical mucus (CM) contact test results. To overcome the deleterious effects of ASAs, techniques such as artificial insemination, intrauterine insemination (IUI), and in vitro fertilization with intracytoplasmic sperm injection are reasonable treatment options [6]. Laboratory techniques including sperm washing, proteolytic enzyme treatment, immunomagnetic sperm separation, and the use of immunobeads are of low efficacy and have yielded conflicting results [7]. Theoretically, IUI bypasses the barrier that the CM poses to ASA-coated sperm. However, sperm washing is still an effective method to decrease ASA load in the semen. Sperm washing removes free antibodies from the seminal plasma but fails to remove sperm-bound antibodies, even after many consecutive washing cycles. This method has similar effectiveness in samples with low and high ASA titers in the native semen [8].
Staphylococcus protein A (SPA) binds to the Fc portion of human immunoglobulins (Igs), a defense mechanism that provides protection from the opsonization that precedes phagocytosis. SPA has a high affinity for the Fc portion of IgG and IgG-containing immune complexes, which can be selectively removed via extracorporeal exposure of seminal plasma to SPA [9]. Immobilized protein A adsorbents have been extensively used for the purification and removal of human IgG from the serum in the treatment of immune-related diseases [10]. This protein has been tested in vivo and has proven successful in the treatment of antibody-induced nephritis, thrombocytopenia, and rheumatoid arthritis [11]. SPA is a highly stable surface factor with a molecular weight of 42 kDa that presents in both secreted and membrane-associated forms [12]. The safety, tolerability, and pharmacokinetics of SPA in animal models, along with encouraging preclinical data, suggest that this protein could be utilized for the treatment of selective autoimmune disorders. The improvement of some autoimmune disorders accompanied by a reduction in autoantibody production supports our proposal to include SPA in new clinical strategies for cases of immunological infertility. We aimed to determine the efficiency of SPA in the reduction of ASA levels and the facilitation of sperm migration in the CM.
Fifteen couples pursuing treatment for infertility who were referred to Al-Hussein Medical City Hospital in Jordan were recruited. Institutional Review Board of King Hussein Medical Center approved this study prior to conducting it. The women were considered to be potentially fertile according to their clinical history. The men, who were the focus of the study, had a history of immunological infertility and the presence of ASAs. Semen samples were obtained via masturbation after 2ŌĆō5 days of abstinence. Upon liquefaction, seminal fluid was subjected to routine semen analysis [13]. Fertile men who consented to participate as semen donors for research purposes were also involved.
CM samples were collected with consent from a number of spontaneously ovulating women during midcycle within a 3-day interval before ovulation. Any medications with potentially negative effects on the rheological characteristics of the mucus were stopped in the previous cycle. Thin, clear CM, with a spinnbarkeit of 10 and a pH > 7.0, was stored in small tubes at 4┬░C and used within 7 days [13]. Only mucus with normal penetration by fertile donor spermatozoa was used for the subsequent tests.
As part of the infertility investigation, a fractional PCT was performed 1 to 3 days prior to the rise in basal temperature. Couples were asked to have intercourse as usual after abstinence for at least 2 days. The CM was evaluated 8ŌĆō12 hours after intercourse and classified as described previously [14]. The PCT result was regarded as adequate when at least two spermatozoa of highly progressive motility and excellent quality per high-power field were counted in the CM; otherwise, it was classified as inadequate.
Seminal fluid samples were centrifuged, and seminal plasma was mixed with dilution buffer (1:50) and centrifuged at 1,000 ├Śg for 10 minutes. A serial dilution of supernatant (1:100, 1:200, and 1:400) was performed. A small aliquot (20 ╬╝L) of the diluted sample was added to 10 ╬╝L of the antigen suspension (Bioserv Diagnostics, Rostock, Germany) and mixed for 2 minutes. Agglutination was considered to occur when sperm antibodies were present in the specimen dilutions of 1:100 and higher.
The classes of Ig present and the precise characterization of the sperm region involved in the autoimmune response were evaluated using indirect immunofluorescence (IF) [15]. Drops of resuspended washed spermatozoa obtained from fertile donors were placed on slides and dried for spermatozoa fixation. Seminal plasma samples were kept on the slides for 1 hour at room temperature, washed twice with phosphate-buffered saline (PBS) (pH 7.2), and incubated with fluorescein isothiocyanate and anti-human IgG, IgM, and IgA for 30 minutes. The slides were then washed again, mounted in a glycerol-buffer mixture, and examined under a fluorescent microscope (Leica Microsystems, Wetzlar, Germany). Positive and negative samples were used in subsequent tests as controls.
Samples underwent liquefaction at room temperature for 30 minutes. Experiments were performed within 1 hour of obtaining the samples. After centrifugation of the seminal fluid at 500 rpm for 10 minutes, the sperm cell pellet was retained and washed twice with sterile PBS (50 mM, pH 7.2). The direct effect of SPA on sperm was tested by mixing washed sperm with different concentrations of SPA (0.156, 0.312, 0.625, 1.25, 2.5, 5, 10, 20, and 40 ╬╝g/mL; Pharmacia, Stockholm, Sweden) in PBS. Freshly prepared vitamin E at a concentration of 25 mM was added, and the mixture was incubated at 37┬░C for 30 minutes [16]. To assess the effect of SPA on sperm viability, two drops of 1% eosin were added, mixed with the sample, and observed under the microscope. Unstained living cells were differentiated from the dead spermatozoa, which stained pink. To study the effect of SPA on sperm motility, a drop of each dilution mixture was placed on a glass slide and checked for immobilization at ├Ś400 magnification. Furthermore, the effect of SPA on the ASA titer was evaluated via mixing with the seminal plasma for 30 minutes at 37┬░C. After incubation, a small portion was used for the latex agglutination test.
In vitro CM penetration was assessed using the capillary tube method [13], in which seminal fluid samples from infertile men (before and after SPA treatment) and seminal fluid samples from fertile men were tested in the same batch. A circular cross-section capillary tube was filled via aspiration using a 1-mL syringe and a plastic tube attached to the upper end of the capillary while the lower end was dipped into a pool of CM. A 7ŌĆō8-cm column of CM was aspirated into the tube so that the upper meniscus was 2ŌĆō3 cm from the top. The top of the tube was sealed, and the lower end was cut off to produce a flat interface. Approximately 200 ╬╝L of the semen sample was placed at the bottom of the small conical plastic and capillary tube, with the open end of this CM-containing tube placed in the semen. Semen reservoirs and capillary tubes were mounted on microscopic slides, placed in a horizontal position in a petri dish, and incubated for 60 minutes at 37┬░C. The slides were removed, and the capillary tube was examined. The length of the tube was scanned to identify the distance farthest from the semen reservoir attained by the spermatozoa. The maximum distance of sperm migration was defined as the migration distance and reported to the nearest 1 cm. Sperm concentrations were assessed at one-half of the migration distance using the same magnification, and spermatozoa were counted while shifting focus from the lower to the upper wall of the capillary in a single pass. Sperm motility was assessed by examining at least 200 spermatozoa at half of the migration distance. Each of the 15 seminal fluid samples was tested at the same time with the same cervical sample. However, to ensure procedural validity, 2 technicians assessed the penetration parameters independently.
SPSS software ver. 13.0 (SPSS Inc., Chicago, IL, USA) was used for data analysis. The results were presented as mean values with standard deviations (SD). Statistical significance of the differences was determined using the t-test, with p-values of < 0.05 considered to indicate significance.
In this prospective study, 15 infertile men with local ASAs were selected. The mean age of these men was 35.67 ┬▒ 3.13 years. With values given as mean ┬▒ SD, the seminal fluid volume was 3.70 ┬▒ 1.26 mL, the pH was 8.125┬▒0.442, the sperm count was 36.27┬▒17.40├Ś106, the percentage of motile sperm (a+b+c) was 33.07%┬▒16.65%, and the percentage of immotile sperm (d) was 66.93%┬▒16.65%. The fraction of normal sperm morphology was 26.40%┬▒8.53%, while that of abnormal sperm morphology was 73.60% ┬▒ 8.53%, and the rate of sperm viability was 57.47% ┬▒ 9.34%. The results revealed a significant positive correlation between ASA titer and sperm immotility (p= 0.005), whereas a significant negative correlation was observed between ASA titer and both the percentage of motile sperm (p= 0.005) and sperm concentration (p= 0.038). The ASA titer had no significant effect on sperm volume, sperm viability, or sperm morphology.
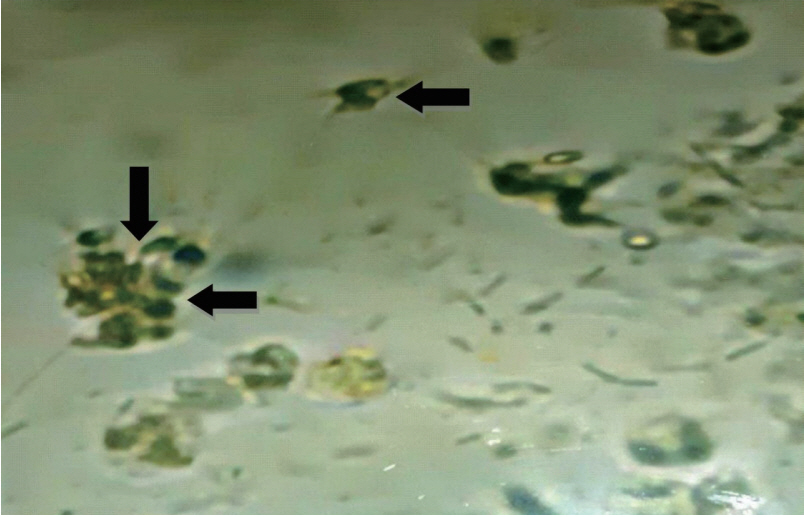
The interactions between sperm and the CM were observed in vivo via PCT, the results of which were inadequate in all female partners (Figure 1). Agglutinated spermatozoa were observed after 8ŌĆō12 hours post-intercourse. Poor PCT results were associated with all semen specimens that displayed positive results for ASA.
The latex agglutination test is recommended for the diagnosis of immunological infertility, as indicated by ASA titers of 100 or higher. In this study, the ASA titer detected in the seminal plasma ranged from 200 to 6,400. In the seminal fluid samples from the infertile men, the IgA class was detected in 20.0%, the IgG class in 6.66%, and a combination of IgA and IgG in 66.7%. Antibodies of the IgM class were detected in one case (6.66%) and were associated with IgG and IgA.
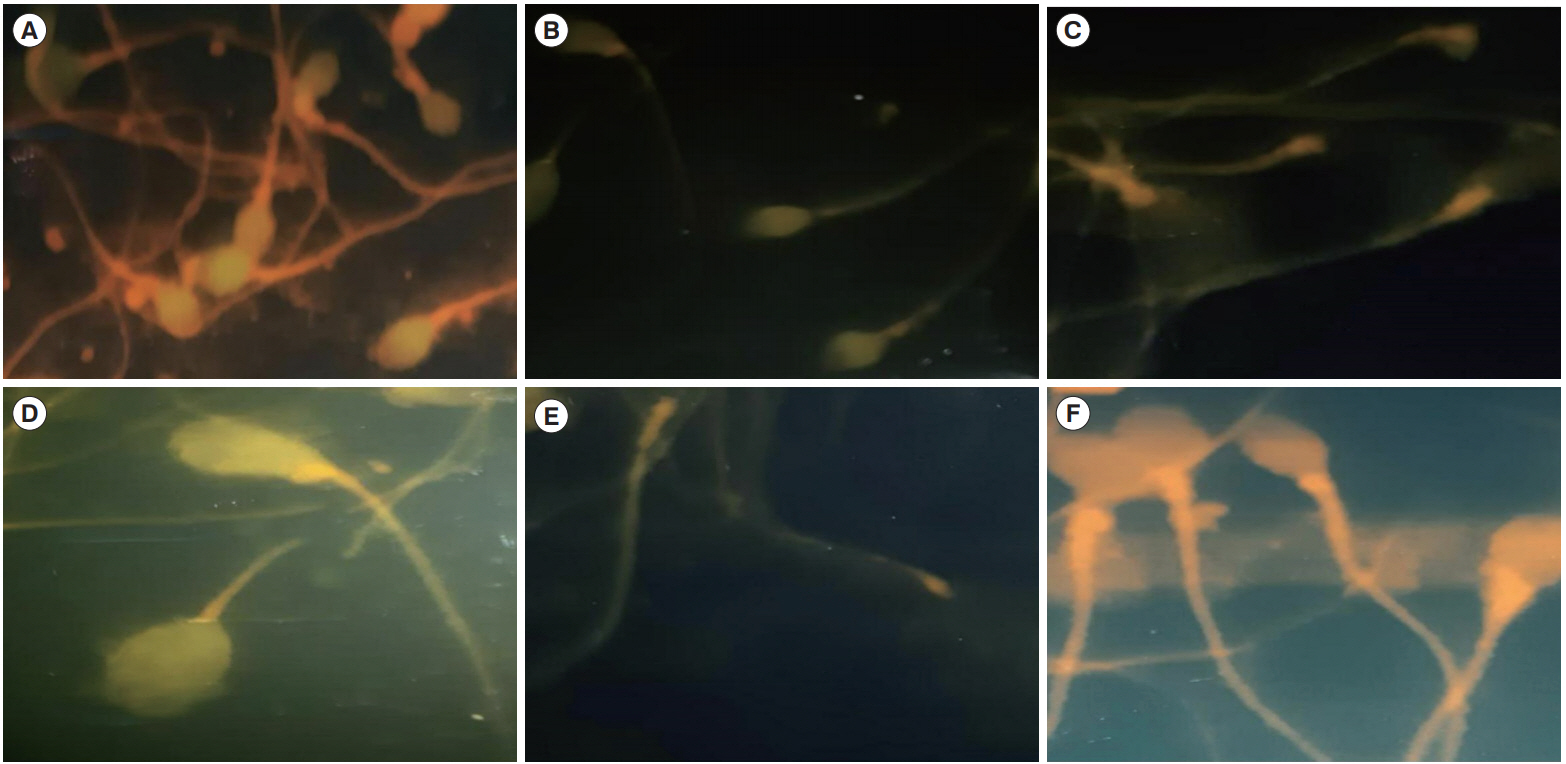
The pattern of immunofluorescent staining as revealed by indirect IF revealed that ASA was associated with the sperm head in four cases (26.6%); the neck in three cases (20%); the head and neck in two cases (13.3%); the tail in two cases (13.3%); and the head, neck, and tail in four cases (26.6%) (Figure 2). The capillary tube method was used to evaluate sperm penetration by ASA-associated sperm. Semen samples with ASAs targeting all sperm parts (the head, the neck, and the tail) showed a significantly lower sperm concentration in the CM (p= 0.015) than samples with ASAs not targeting all regions. However, ASAs targeted specifically to the neck segment were associated with a significantly shorter migration distance in the CM than ASAs to the tail or head segments (p= 0.029).
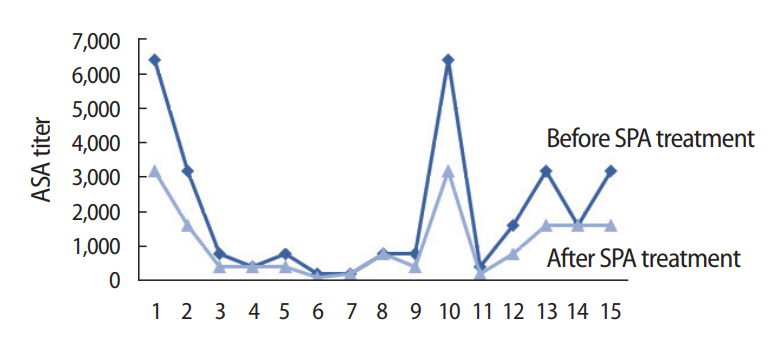
Seminal fluid samples were treated with various dilutions of purified SPA in PBS and analyzed for sperm motility and ASA titer. A concentration of SPA displaying no immobilization effect on motile spermatozoa (range, 2.5ŌĆō10 ┬Ąg/100 mL) was used for the cervical penetration test. Sperm parameters and ASA titer were recorded post-incubation with SPA for 30 minutes at 37┬░C (Figure 3). From pretreatment to posttreatment, the mean ASA titer significantly decreased from 3,200 to 1,600, constituting a 50% decrease.
SPA-treated seminal fluid was assessed regarding mucus penetration using the capillary tube penetration method. Sperm penetration was quantified by measuring the migration distance (the maximum distance of capillary migration from a semen reservoir by spermatozoa after 1 hour), the sperm concentration at half of the migration distance, and the progressive motility of spermatozoa. The values were recorded as mean ┬▒SD (Fig. 4). From pre- to posttreatment, the ASA-positive seminal fluid samples showed significant differences in migration distance (1.54 ┬▒1.1 cm and 101 ┬▒93.7 cm, respectively; p<0.001) and sperm concentration (2.6 ┬▒1.4 and 174.0 ┬▒121.0, respectively; p=0.033) (Table 1). No significant differences (p=0.066) were evident in the number of spermatozoa with progressive motility that penetrated the CM (15.0┬▒9.8 and 27.0┬▒16.0, respectively). Treated semen samples revealed higher numbers of sperm, which allowed high-quality sperm to travel a relatively long distance in the CM.
Aberrant immune homeostasis may give rise to immunological infertility. ASAs appear to cause infertility through sperm agglutination, immobilization, or opsonization, all of which impair sperm transportation through the female reproductive tract; reduce the number of spermatozoa at the fertilization point; impair the sperm-egg interaction, acrosome reaction, and binding to the zona pellucida; and eventually affect the fertilization rate [17]. However, not all ASAs can alter sperm function, as the cognate antigen may not be associated with the fertilization process or the antibodies may not bind to the functional domain of the antigen [18]. World Health Organization guidelines (1992) indicate that significant quantities of ASAs belonging to the IgG or IgA class can impact the function of fertility antigen-1 [19].
ASAs in infertile men usually appear in the seminal fluid as multiple classes of Ig. In this study, IgA was present as the only isotype in the seminal fluid of only 20% of the infertile men. IgM seems to have no clinical impact, as it is rarely detected either alone or in combination with IgA or IgG. Our results align with previous findings confirming the relatively strong impact of the combination of IgG and IgA directed toward various sites of the spermatozoa [6,20]. Although the IgA isotype appeared more clinically significant than the IgG isotype, it is rarely present without associated IgG. From a biological standpoint, IgA seems to be the most important Ig in this context, and its presence on the sperm surface significantly impairs sperm progression through the CM [21]. In vasectomized men, previous results have shown that immune responses restricted to the IgG class are not rare, but those men appeared to have normal fertility, whereas the IgA response was inversely correlated with the fertility rate [22].
ASAs can adhere to various sites on the sperm regardless of subclass [23]. Those that adhere to the tails of sperm can cause sperm immobilization or clumping, whereas ASAs that associate with the head inhibit the penetration of CM by sperm [24]. Immunity to sperm antigens is restricted to certain sperm surface region. In this study, seminal fluid with ASAs associated with the neck region showed a significant decrease in migration distance in the CM contact test. Therefore, neck-associated ASAs seem to be involved in infertility [25]. However, in 1988, Clarke et al. [26] reported a significant association between poor penetration and the presence of antibodies on the sperm tail main piece. The concentration of spermatozoa and the linear velocity of progression influenced the number of spermatozoa penetrating the mucus in unit times, presumably by impacting the frequency of collisions at the CM interface [27]. When ASAs are present in the semen or the CM, an interaction can occur between galactose residues on the spermatozoa and galactose recognition sites on the ASAs. In addition, binding can also occur between the Fc region of the antibodies and the CM [28].
ASAs are known to play a role in male and female reproductive failure. In many cases, they are thought to be a relative rather than an absolute cause of infertility. It has been reported that the pregnancy rate from artificial insemination is higher if the mucus penetration test yields normal results. Several strategies have been used in an effort to improve the potentially deleterious effects of ASA-mediated infertility. These include reductions in ASA production, removal of ASAs via the conventional swim-up procedure, washing of spermatozoa, and separation techniques based on principles such as migration, filtration, or density gradient centrifugation [6]. However, no method to counteract ASAs has been universally accepted. Some reports have been published evaluating the use of specific IgA proteases from Neisseria species on sperm-bound Ig. Sperm coated with IgA were found to show improved penetration in CM after IgA protease exposure. The use of intravaginal protease has been suggested as a treatment for CM antibodies [29,30].
It has been reported that Staphylococcus aureus, which has been frequently isolated from patients with male genitourinary infections, produces extracellular products that adversely impact human sperm motility [31]. To overcome the deleterious effect of S. aureus on sperm, chemical supplements have been applied to improve sperm parameters and inhibit sperm immobilization by Staphylococcal products [16]. Prohibition of Staphylococcus-induced sperm immobilization was observed in pre-incubation seminal fluid with vitamin B1, vitamin E, glutathione, and sodium selenite. Therefore, in the present study, vitamin E was used as an adjuvant to protect the spermatozoa and help maintain their movement when incubated with SPA. The SPA protein was able to dramatically reduce the amount of seminal plasma-derived Egg and IgA antibodies by 50%ŌĆō75%. Many of the effects of Igs are mediated via the Fc region; hence, the binding of the Fc region by SPA may be relevant.
Although Staphylococcus species were the most common bacteria isolated from asymptomatic men undergoing fertility evaluation, the bacterial count has been found to be uncorrelated with the semen parameters [32]. In the present study, a proposed in vitro treatment for ASA was applied in which SPA was used as a means of masking antibodies in the seminal fluid samples. In each case, ASA titer was significantly reduced posttreatment with SPA. The immunomodulatory properties of SPA in binding the Fc fragment of Igs, as well as the capability of SPA to bind the Fab regions of the B-cell receptor and promote B-cell apoptosis (leading to the inhibition of autoantibody production), have drawn attention to the possibility of using this protein to facilitate the elimination of sperm antibodies. SPA is a highly stable cell surface receptor that contains 5 homologous Ig-binding domains. This protein is capable of binding to the Fc region of Igs, especially IgGs, from a large number of species. One protein A molecule has been shown to simultaneously bind at least two molecules of IgG. Protein A binds to the Fc region of human IgG, IgM, IgA, IgE, IgG2a, and IgG2b subclasses [33]. Our results illustrate that SPA binds IgG and IgA molecules through the Fc region, thereby reducing the number of Fc sites available for sperm binding and enhancing sperm penetration in the CM. The numbers of sperm that were successful in reaching the deepest point in the CM were significantly increased after SPA treatment. However, the presence of certain receptors for IgG (Fc) on the sperm surface leads to a competition between SPA and sperm for binding of the Fc portion, with a higher tendency and affinity of SPA-IgG Fc binding (4├Ś107ŌĆō2├Ś108 moles/L) [34].
Although the number of sperm with fast progressive movement in the CM did not significantly increase (p= 0.066) after SPA treatment, the number of sperm that deeply penetrated the mucus did increase significantly. Experimental studies have suggested that the Fc region of IgA binds receptors within the mucus, impairing progressive forward motility of the sperm [35]. Men with positive sperm antibody tests but with a sperm cervical penetration value of > 1 cm at 1 hour produce pregnancies in their partners at reasonable rates, whereas those with no mucus penetration have a very poor prognosis for pregnancy [36].
The observation of in vitro sperm penetration provides reliable data for fertility assessment and allows greater discrimination of sperm function than semen analysis alone. Our results indicate that sperm processing before IUI should be attempted before starting the more invasive and expensive techniques of assisted reproduction. The SPA processing technique improves sperm quality with regard to concentration and penetration ability. The study could be helpful to andrologists and embryologists who are seeking to optimize sperm quality in men with autoantisperm antibodies. In spite of the significant reduction in ASAs present in seminal fluid following SPA treatment, we did not test the effectiveness of the post-sperm pools in IUI. Therefore, caution should be applied to the interpretation of our findings until well-designed clinical trials have confirmed the effectiveness of SPA in eluting ASAs and increasing the chances for conception in couples with unexplained infertility. As such, the influence of SPA on pregnancy rate requires further study.
Acknowledgments
The author would like to express appreciation to Al-Balqa Applied University for supporting this work.
Figure┬Ā1.
Postcoital test that revealed head to head sperm agglutination (arrows) in the cervical mucus of the female (├Ś40).
Figure┬Ā2.
Identification of sperm part targeted by antisperm antibody (ASA) using indirect immunofluorescence staining ( ├Ś 100). (A) Green to yellow sperm head means that the antibody was directed to the head region of the sperm. (B) ASA directed to the head, neck, and tail. (C) ASA directed to the neck and tail. (D) ASA directed to the head and tail. (E) ASA directed to the tail. (F) All sperm parts showed red color which means the absence of anti-sperm antibody. Green to yellow color means positive, and red color means negative. Three parts of sperm could be a target for antisperm antibody: head, neck, and tail.
Figure┬Ā3.
Mean of Staphylococcus protein A (SPA) on antisperm antibody titer in seminal fluid samples. ASA, antisperm antibody.
Figure┬Ā4.
Cervical mucus penetration results by seminal fluid with antisperm antibody before and after Staphylococcus protein A treatment with respect to the (A) migration distance, (B) progressive motility, and (C) sperm concentration.

Table┬Ā1.
Cervical penetration test results of seminal fluid samples before and after SPA treatment with respect to migration distance, sperm concentration, and progressive motility
References
1. Roupa Z, Polikandrioti M, Sotiropoulou P, Faros E, Koulouri A, Wozniak G, et al. Causes of infertility in women at reproductive age. Health Sci J 2009;3:80-7.
2. Kipersztok S, Kim BD, Morris L, Drury KC, Williams RS, Rhoton-Vlasak A. Validity of a rapid assay for antisperm antibodies in semen. Fertil Steril 2003;79:522-8.


3. Hossain A, Islam N, Aryal S, Madanes A. The prevalence of circulating anti sperm antibody (ASA) in infertile population representing of all etiologies. Middle East Fertil Soc J 2007;12:27-30.
4. Brazdova A, Vermachova M, Zidkova J, Ulcova-Gallova Z, Peltre G. Immunodominant semen proteins I: new patterns of sperm proteins related to female immune infertility. Cent Eur J Biol 2013;8:813-8.


5. Wang M, Shi JL, Cheng GY, Hu YQ, Xu C. The antibody against a nuclear autoantigenic sperm protein can result in reproductive failure. Asian J Androl 2009;11:183-92.



6. Felemban A, Hassonah SM, Felimban N, Alkhelb H, Hassan S, Alsalman F. Sperm surface antibodies: IUI vs. IVF treatment. Obstet Gynecol Res 2018;1:80-4.

7. Naz RK. Modalities for treatment of antisperm antibody mediated infertility: novel perspectives. Am J Reprod Immunol 2004;51:390-7.


8. Schneider D, Feijo C, Verza Jr S, Esteves S. Effectiveness of sperm washing by discontinuous density gradient centrifugation to remove antibodies bound to the sperm membrane. Med Express 2014;1:123-6.

9. Silverman GJ, Goodyear CS, Siegel DL. On the mechanism of staphylococcal protein A immunomodulation. Transfusion 2005;45:274-80.


10. Jia L, Yang L, Zou H, Zhang Y, Zhao J, Fan C, et al. Protein A tangential flow affinity membrane cartridge for extracorporeal immunoadsorption therapy. Biomed Chromatogr 1999;13:472-7.


11. Eftimiadi G, Vinai P, Eftimiadi C. Staphylococcal protein A as a pharmacological treatment for autoimmune disorders. J of Autoimmune Disorders 2017;3:40.
12. OŌĆÖHalloran DP, Wynne K, Geoghegan JA. Protein A is released into the Staphylococcus aureus culture supernatant with an unprocessed sorting signal. Infect Immun 2015;83:1598-609.



13. World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge: Cambridge University Press; 1999.

14. Eggert-Kruse W, Gerhard I, Tilgen W, Runnebaum B. Clinical significance of crossed in vitro sperm-cervical mucus penetration test in infertility investigation. Fertil Steril 1989;52:1032-40.


15. Hjort T, Hansen KB. Immunofluorescent studies on human spermatozoa. I. The detection of different spermatozoal antibodies and their occurrence in normal and infertile women. Clin Exp Immunol 1971;8:9-23.


16. Gupta S, Rana M, Thaper D, Arora SK, Prabha V. Impact of chemical supplementation on the impairment of sperm parameters induced by sperm immobilizing factor. Int J Curr Microbiol App Sci 2014;3:871-80.
17. Chiu WW, Chamley LW. Clinical associations and mechanisms of action of antisperm antibodies. Fertil Steril 2004;82:529-35.


18. Bohring C, Krause W. Immune infertility: towards a better understanding of sperm (auto)-immunity. The value of proteomic analysis. Hum Reprod 2003;18:915-24.



19. World Health Organization. WHO laboratory manual for the examination of human semen and semen-cervical mucus interaction. 3rd ed. Cambridge: Cambridge University Press; 1992.
20. Ulcova-Gallova Z. Infertility attack of immunity. 1st ed. Prague: Grada Publishing Prague; 2006.
22. Meinertz H, Linnet L, Fogh-Andersen P, Hjort T. Antisperm antibodies and fertility after vasovasostomy: a follow-up study of 216 men. Fertil Steril 1990;54:315-21.


23. Turek PJ. Immunopathology and infertility. In: Lipshultz LI, Howards SS, editors. Infertility in the male. St. Louis: Mosby-Yearbook; 1997. p. 305-25.
24. Agostini A, Lucas H. Semen analysis. 9th Postgraduate course for training in reproductive medicine and reproductive biology. Geneva: 2005.
25. Mahdi BM, Salih WH, Caitano AE, Kadhum BM, Ibrahim DS. Frequency of antisperm antibodies in infertile women. J Reprod Infertil 2011;12:261-5.


26. Clarke GN, Hyne RV, du Plessis Y, Johnston WI. Sperm antibodies and human in vitro fertilization. Fertil Steril 1988;49:1018-25.


27. Aitken RJ, Sutton M, Warner P, Richardson DW. Relationship between the movement characteristics of human spermatozoa and their ability to penetrate cervical mucus and zona-free hamster oocytes. J Reprod Fertil 1985;73:441-9.


28. Chantler E, Sharma R, Sharman D. Changes in cervical mucus that prevent penetration by spermatozoa. Symp Soc Exp Biol 1989;43:325-36.

29. Kutteh WH, McAllister D, Byrd W, Mestecky J. Antisperm antibodies: current knowledge and new horizons. Mol Androl 1992;4:183-93.
30. Kutteh WH, Kilian M, Ermel LD, Mestecky J. Antisperm antibodies in infertile women: subclass distribution of immunoglobulin (Ig) A antibodies and removal of IgA sperm-bound antibodies with a specific IgA1 protease. Fertil Steril 1995;63:63-70.


31. Gupta S, Prabha V. Human sperm interaction with Staphylococcus aureus: a molecular approach. J Pathog 2012;2012:816536.




32. Rodin DM, Larone D, Goldstein M. Relationship between semen cultures, leukospermia, and semen analysis in men undergoing fertility evaluation. Fertil Steril 2003;79 Suppl 3:1555-8.


33. Graille M, Stura EA, Corper AL, Sutton BJ, Taussig MJ, Charbonnier JB, et al. Crystal structure of a Staphylococcus aureus protein A domain complexed with the Fab fragment of a human IgM antibody: structural basis for recognition of B-cell receptors and superantigen activity. Proc Natl Acad Sci U S A 2000;97:5399-404.



34. Kessler SW. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol 1975;115:1617-24.



- TOOLS






