 |
 |
- Search
| Clin Exp Reprod Med > Volume 41(3); 2014 > Article |
Abstract
Objective
This study was designed to investigate the survival rate of vitrified mouse blastocysts depending on the presence or absence of sucrose in vitrification solution.
Methods
Mouse two-cell embryos were collected and cultured to blastocysts. Two vitrification solutions were prepared. The control solution was composed of 25% glycerol, 25% ethylene glycol, and 0.5 M sucrose (G25E250.5S) containing 2.5 mL glycerol, 2.5 mL ethylene glycol, 2 mL SSS, and 0.855 g sucrose in 5 mL PB1. The experimental solution was composed of 25% glycerol and 25% ethylene glycol (G25E25) and contained 2.5 mL glycerol and 2.5 mL ethylene glycol in 5 mL PB1. Artificial shrinkage was conducted by aspirating the blastocoelic fluid using an ICSI pipette. To examine the effect of sucrose in the vitrification solution on the survival rate of mouse blastocysts, the shrunken-equilibrated blastocysts were rehydrated or vitrified after being exposed to one of the two vitrification solutions. After exposure and the vitrification-thawing process, the re-expansion rate and hatching rate were evaluated after 6 hours of in vitro culture.
Results
The re-expansion rate of mouse blastocysts exposed to vitrification solution with and without sucrose were not different in the experimental solution (without sucrose) (98%) and the control solution (with sucrose) (92%) (p>0.05). The hatching rate was higher in the experimental solution (95%) than in the control solution (88%), but did not differ across two treatments (p>0.05). The re-expansion rate of mouse blastocysts vitrified in the control solution was 92% and 94%, respectively (p>0.05), and the hatching rate was higher in the experimental solution (90%) than in the control solution (74%) (p<0.05).
The increased speed of developing embryos to the blastocyst stage through the development of continuous culture media and improved culture conditions has resulted in the transfer of blastocysts on day 5 after ovum retrieval instead of an 8-cell embryo on day 3 [1,2]. Furthermore, because cryopreservation of surplus blastocysts can reduce the burden on infertile patients and increase the chances of pregnancy, research has focused on improving the survival rate of the vitrified blastocysts after thawing [3,4,5,6].
Slow freezing was once used for blastocyst cryopreservation [7]. During slow freezing, crystallization occurs abundantly, with resulting relatively low blastocyst preservation. To overcome these problems, vitrification is now applied to freeze blastocysts [8,9,10,11,12,13,14,15].
Cohen et al. [7] first reported the use of slow freezing cryopreservation of human blastocysts. Vitrification for human blastocysts was introduced by Vanderzwalmen et al. [12] in 1992. Initially, the survival rate of frozen-thawed human blastocysts was also very disappointing. One contributing factor was intracellular and extracellular ice crystal formation due to inco mplete dehydration [11,16,17]. Another factor was osmotic injury caused by the high concentration of vitrification solution and the long equilibration time for dehydration, due to the relatively huge volume of the blastocoele cavity [11,16,17]. These two factors decreased the survival rate after thawing. Lastly, the in vitro culture and freezing process can produce zona hardening, which lowers the hatching rate after thawing [11,18,19].
To overcome these problems, artificial shrinkage, assisted hatching, and special containers have been developed. Artificial shrinkage is a method in which the fluid of the blastocoele is aspirated artificially using a micro-pipette, 29-guage needle, hand-drawn Pasteur pipette, or micro-needle [20,21,22,23]. Assisted hatching has been conducted by laser or mechanically using a micro-pipette. After assisted hatching, the embryos are contained by specialized containers such as the Hemi-straw, EM-grid, cryotop, or cryoloop [24,25,26,27]. These refinements have improved the survival rate of the vitrified blastocysts and the pregnancy rate.
Sucrose can be included in the vitrification solution to dehydrate free water and protect the cell membrane. However, blastocoelic fluid has already been aspirated from an artificially shrunken blastocyst, and the tightly condensed condition of an artificially shrunken blastocyst is very similar to a somatic cell. Therefore, sucrose for dehydration may not need to be added to the vitrification solution used for artificially shrunken blastocysts.
This study was conducted to evaluate the survival rate of vitrified mouse blastocysts depending on the addition or omission of sucrose from the vitrification solution.
Five-week-old female mice (C57BL/CBA) were superovulated by an intraperitoneal injection of 5 IU of pregnant mare serum gonadotropin (PMSG) followed 48 hours later by 5 IU of human chorionic gonadotropin (hCG, Sigma-Aldrich, St. Louis, MO, USA) and immediately paired with males of the same strain. On the following morning, mating was confirmed by checking for a vaginal plug. Forty-eight hours after hCG injection, two-cell embryos were collected and cultured in groups of 10 in 30 ┬ĄL drops of medium under mineral oil. All embryos were cultured to the 8-cell stage in G1.1 culture medium (Vitrolife, Goteborg, Sweden) and to the blastocyst stage in G2.2 culture medium (Vitrolife).
Equilibration solutions consisted of EBS1 (10% glycerol) and EBS2 (10% glycerol+20% ethylene glycol). EBS1 contained 1 mL glycerol, 2 mL serum substitute supplement (SSS, Irvine, Irvine, CA, USA), and 7 mL phosphaste buffer 1 (PB1, Gibco, San Diego, CA, USA). EBS2 contained 1 mL glycerol, 2 mL ethylene glycol, 2 mL SSS, and 5 mL PB1. For vitrification, two different solutions were prepared. The control solution was composed of 25% glycerol, 25% ethylene glycol 25%, and 0.5 M sucrose and contained 2.5 mL glycerol, 2.5 mL ethylene glycol, 2 mL SSS, and 0.855 g sucrose in 5 mL PB1. The experimental solution was composed of 25% glycerol and 25% ethylene glycol and contained 2.5 mL glycerol and 2.5 mL ethylene glycol in 5 mL PB1. The thawing solution was composed of sucrose solution (0.5 M, 0.25 M, and 0.125 M), PB1, and 20% SSS.
The blastocysts were fixed with a holding pipette after turning the inner cell mass (ICM) to 6 or 12 o'clock. Then, an intracytoplasmic sperm injection pipette was inserted into the blastocoelic cavity and about 70% to 80% of the blastocoelic fluid was aspirated (Figure 1). After artificial shrinkage, the shrunken blastocyst was equilibrated in 10% glycerol and 10% glycerol+20% ethylene glycol solution for 3 minutes at room temperature, in sequence, and transferred to the vitrification solution. After 10 seconds, the blastocysts of the control group were re-equilibrated and the blastocysts of the experimental group were loaded in a capped-pulled straw and frozen [28]. After seven days of cryopreservation, for the thawing process, blastocysts were rehydrated with 0.5 M, 0.25 M, and 0.125 M sucrose for 3 minutes at room temperature, in sequence and rinsed with PB1 3 times. Assisted hatching was then performed (partial zona dissection). After fixing the embryo using a holding pipette, a hand-made hatching pipette was inserted in the perivitelline space and penetrated opposite the zona pellucida. The zona was split with holding and assisted hatching pipettes (partial zona dissection, Figure 2). The re-expansion rate and hatching rate were evaluated 6 hours after re-hydration or thawing. These procedures were repeated 5 times. In each experiment, 10 blastocysts of each group were observed. The results were analyzed with the Student's t-test.
After the equilibration of the artificially shrunken blastocysts, they were exposed to different vitrification solutions. Ten seconds later, rehydration was conducted without freezing. The aim of this experiment was to determine whether the absence of sucrose in the vitrification solution was harmful to the shrunken blastocysts. After 6 hours of culture, the re-expansion rate and hatching rate of the blastocysts exposed to vitrification solution with sucrose (control) were 92% and 88%, respectively. The re-expansion rate and hatching rate of the blastocysts exposed to vitrification solution without sucrose (experiment) were 98% and 95%, respectively. There was no statistically significant difference (Table 1).
The re-expansion rate and hatching rate of the vitrified blastocysts were 92% and 74% in the presence of sucrose (control), and 94% and 90% in the absence of sucrose (experiment), respectively. There was a statistically significant difference in the hatching rate between the two groups (p=0.037) (Table 2).
Sucrose is a cryoprotectant and is used for several purposes during the freezing process. It is used for intracellular water removal to prevent ice crystal formation and also to protect the cell membrane and cytoplasm when the influx of highly concentrated permeating cryoprotectants into the cytoplasm occurs rapidly [29,30,31,32,33].
Sucrose is also used during the thawing process. While thawing in sucrose solution, the removal of the cryoprotectant and rehydration in cytoplasm can make the shrunken blastocysts re-expand [34,35,36,37,38].
The survival rate and hatching rate after thawing are affected by several factors such as ice crystal formation, shifting of blastocoelic fluid, and zona hardening. During the vitrification of blastocysts, if insufficient blastocoelic fluid is released, ice crystal formation inside the blastocoeles would occur extensively and the blastocysts would be severely damaged [11,16,17]. Also, during dehydration, the trophoblast cell might be damaged by the shifting of a large amount of water [11,16,17]. Zona hardening that occurs during in vitro culture and the freezing procedure affects the hatching rate [18,19].
Recently, to overcome low survival and hatching rates, several studies have been conducted. Martino et al. [24] attempted to use ultra-rapid cooling for cryopreservation. Researchers have also tried to use variations of the vitrification method such as the use of a hemi-straw carrier [26] or containerless vitrification [25]. Other studies have suggested that artificial shrinkage could increase the survival rate by preventing ice crystal formation [20,21,22,23].
Usually, the vitrification solution contains 30% to 40% of the permeating cryoprotectant and a non-permeating protectant such as Ficoll and sucrose. However, in this study, the vitrification solution contained 50% of the permeating cryoprotectant and Ficoll was not added because ice formation did not occur with higher concentrations of the permeating cryoprotectant. Sucrose, which is used for dehydration, also was not added to the vitrification solution in this study, because the blastocysts were already shrunken artificially.
Similar vitrification solutions, that is, those without sucrose, have been used for the vitrification of somatic cells such as stem cells. Because the cell size is relatively small and the amount of water in the cytoplasm is small in stem cells or somatic cells compared to an embryo, the cell membrane was not significantly damaged by dehydration.
The blastocyst is composed of the trophoectoderm and ICM. The shrunken blastocyst may be morphologically similar to somatic cells. Therefore, we hypothesized that sucrose may not need to be added to the vitrification solution in the case of shrunken blastocysts. The results of this study suggest that this hypothesis may be correct.
Additionally, sucrose may react more efficiently in the thawing process. During the thawing process, the blastocyst is first exposed to sucrose solution, and this exposure can make rehydration more effective.
In this study, the re-expansion rate was not statistically different in either group, but was higher in the group that was exposed to vitrification solution without sucrose. However, the hatching rate was significantly higher in the group exposed to vitrification solution without sucrose (p=0.037). This result suggests that blastocysts in vitrification solution without sucrose react more sensitively to a sucrose solution in the thawing process.
In conclusion, sucrose has been used for several decades in the cryopreservation of embryos, but when the artificial shrinkage metnhod was used, the survival rate of mouse blastocysts was higher in the absence of sucrose than in the presence of sucrose [39]. In our clinic, we have used vitrification solution without sucrose in the cryopreservation of human blastocysts, and the outcomes are better than when vitrification solution with sucrose is used. Therefore, we think that further research should be carried out on effects of sucrose on the survival rate of the vitrified blastocysts.
References
1. Gardner DK, Schoolcraft WB, Wagley L, Schlenker T, Stevens J, Hesla J. A prospective randomized trial of blastocyst culture and transfer in in-vitro fertilization. Hum Reprod 1998;13:3434-3440.PMID: 9886530.


2. Jones GM, Trounson AO, Gardner DK, Kausche A, Lolatgis N, Wood C. Evolution of a culture protocol for successful blastocyst development and pregnancy. Hum Reprod 1998;13:169-177.PMID: 9512252.


3. Menezo Y, Nicollet B, Herbaut N, Andre D. Freezing cocultured human blastocysts. Fertil Steril 1992;58:977-980.PMID: 1426385.


4. Bonsi A. Blastocyst transfer and freezing: can this help us to improve the success of assisted reproduction? Singapore J Obstet Gynecol 1995;26:13-17.
5. Kaufman RA, Menezo Y, Hazout A, Nicollet B, DuMont M, Servy EJ. Cocultured blastocyst cryopreservation: experience of more than 500 transfer cycles. Fertil Steril 1995;64:1125-1129.PMID: 7589664.


6. Nakayama TGY, Kanzaki H, Takabatake K, Himeno T, Takakura K, et al. Cryopreservation of human blastocyst In: IX world congress on in vitro Fertilization and Alternated Assisted Reproduction; 1995 April 3-7; Vienna, Austria. pp 451-454.
7. Cohen J, Simons RF, Edwards RG, Fehilly CB, Fishel SB. Pregnancies following the frozen storage of expanding human blastocysts. J In vitro Fert Embryo Transf 1985;2:59-64.


8. Saito N, Imai K, Tomizawa M. Effect of sugars-addition on the survival of vitrified bovine blastocysts produced in vitro. Theriogenology 1994;41:1053-1060.PMID: 16727458.


9. Yang NS, Lu KH, Gordon I, Polge C. Vitrification of blastocysts produced in vitro. Theriogenology 1992;37:326.

10. Fahy GM, MacFarlane DR, Angell CA, Meryman HT. Vitrification as an approach to cryopreservation. Cryobiology 1984;21:407-426.PMID: 6467964.


11. Zhu SE, Kasai M, Otoge H, Sakurai T, Machida T. Cryopreservation of expanded mouse blastocysts by vitrification in ethylene glycol-based solutions. J Reprod Fertil 1993;98:139-145.PMID: 8345457.


12. Vanderzwalmen P, Delval A, Chatziparasidou A, Bertin G, Ectors F, Lejeune B, et al. Pregnancies after vitrification of human day 5 embryos. Hum Reprod 1997;12(Suppl): 98.

13. Van Der Zwalmen P, Gaurois B, Ectors FJ, Touati K, Massip A, Ectors F. Some factors affecting successful vitrification of mouse blastocysts. Theriogenology 1988;30:1177-1183.PMID: 17087907.


14. Menezes DM, Orth D. Comparative analysis of changes resulting from bite plate therapy and Begg treatment. Angle Orthod 1975;45:259-266.PMID: 1059338.

15. Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryos at -196 degrees C by vitrification. Nature 1985;313:573-575.PMID: 3969158.


16. Scheffen B, Van Der Zwalmen P, Massip A. A simple and efficient procedure for preservation of mouse embryos by vitrification. Cryo Letters 1986;7:260-269.
17. Miyake T, Kasai M, Zhu SE, Sakurai T, Machida T. Vitrification of mouse oocytes and embryos at various stages of development in an ethylene glycol-based solution by a simple method. Theriogenology 1993;40:121-134.PMID: 16727299.


18. Carroll J, Depypere H, Matthews CD. Freeze-thaw-induced changes of the zona pellucida explains decreased rates of fertilization in frozen-thawed mouse oocytes. J Reprod Fertil 1990;90:547-553.PMID: 2250252.


19. Germond M, Senn A, Rink K, Delacr├®tas G, De Grandi P. Is assisted hatching of frozen-thawed embryos enhancing pregnancy outcome in patients who had several previous indation failures? J Reprod Fertil 1995;3:41-42.
20. Vanderzwalmen P, Bertin G, Debauche C, Standaert V, van Roosendaal E, Vandervorst M, et al. Births after vitrification at morula and blastocyst stages: effect of artificial reduction of the blastocoelic cavity before vitrification. Hum Reprod 2002;17:744-751.PMID: 11870130.


21. Son WY, Yoon SH, Yoon HJ, Lee SM, Lim JH. Pregnancy outcome following transfer of human blastocysts vitrified on electron microscopy grids after induced collapse of the blastocoele. Hum Reprod 2003;18:137-139.PMID: 12525454.


22. Hiraoka K, Hiraoka K, Kinutani M, Kinutani K. Blastocoele collapse by micropipetting prior to vitrification gives excellent survival and pregnancy outcomes for human day 5 and 6 expanded blastocysts. Hum Reprod 2004;19:2884-2888.PMID: 15347597.


23. Mukaida T, Oka C, Goto T, Takahashi K. Artificial shrinkage of blastocoeles using either a micro-needle or a laser pulse prior to the cooling steps of vitrification improves survival rate and pregnancy outcome of vitrified human blastocysts. Hum Reprod 2006;21:3246-3252.PMID: 16936299.


24. Martino A, Songsasen N, Leibo SP. Development into blastocysts of bovine oocytes cryopreserved by ultra-rapid cooling. Biol Reprod 1996;54:1059-1069.PMID: 8722627.


25. Lane M, Bavister BD, Lyons EA, Forest KT. Containerless vitrification of mammalian oocytes and embryos. Nat Biotechnol 1999;17:1234-1236.PMID: 10585728.


26. Vanderzwalmen P, Bertin G, Debauche C, Standaert V, Bollen N, van Roosendaal E, et al. Vitrification of human blastocysts with the Hemi-Straw carrier: application of assisted hatching after thawing. Hum Reprod 2003;18:1504-1511.PMID: 12832379.


27. Kuwayama M, Vajta G, Ieda S, Kato O. Comparison of open and closed methods for vitrification of human embryos and the elimination of potential contamination. Reprod Biomed Online 2005;11:608-614.PMID: 16409712.


28. Jo DH, Ko GR, Jung JH, Choi JR, Joo JK, Lee KS. Effects of the artificial shrinkage and assisted hatching before vitrification on the development of the vitrified mouse expanding blastocysts. Korean J Reprod Med 2008;35:275-283.
29. Willadsen SM, Polge C, Rowson LE, Moor RM. Deep freezing of sheep embryos. J Reprod Fertil 1976;46:151-154.PMID: 1271336.


30. Whittingham DG, Wood M, Farrant J, Lee H, Halsey JA. Survival of frozen mouse embryos after rapid thawing from -196 degrees C. J Reprod Fertil 1979;56:11-21.PMID: 469830.


31. Rall WF, Reid DS, Farrant J. Innocuous biological freezing during warming. Nature 1980;286:511-514.PMID: 7402331.


32. Smorag Z, Katska L, Wierzchos E. Some factors affecting the viability of mouse and cattle embryos frozen to -40 deg C before transfer to liquid nitrogen. Anim Reprod Sci 1981;4:65-72.

33. Massip A, Vanderzwalmen P. In vitro survival of mouse embryos frozen in glycerol or glycerol-sucrose. Cryo Letters 1982;3:326.
34. Szell A, Shelton JN. Osmotic and cryoprotective effects of glycerol-sucrose solutions on day-3 mouse embryos. J Reprod Fertil 1987;80:309-316.PMID: 3598965.


35. Szell A, Shelton JN. Sucrose dilution of glycerol from mouse embryos frozen rapidly in liquid nitrogen vapour. J Reprod Fertil 1986;76:401-408.PMID: 3944806.


36. Willadsen S, Polge C, Rowson LE. The viability of deep-frozen cow embryos. J Reprod Fertil 1978;52:391-393.PMID: 633224.


37. Friedler S, Giudice LC, Lamb EJ. Cryopreservation of embryos and ova. Fertil Steril 1988;49:743-764.PMID: 3282929.


38. Leibo SP. Principles of cryopreservation for ART laboratory In: Proceeding for 13th annual postgraduate program for ASRM. Course VII. Current techniques and new prontiers in cryopreservation and micromanipulation; 1997;pp 1-14.
39. Sun ST, Choi JR, Son JB, Joo JK, Ko GR, Lee KS. The effect of long zona dissection using ICSI pipettes for mechanical assisted hatching in vitrified-thawed blastocyst transfers. J Assist Reprod Genet 2012;29:1431-1434.PMID: 23054363.



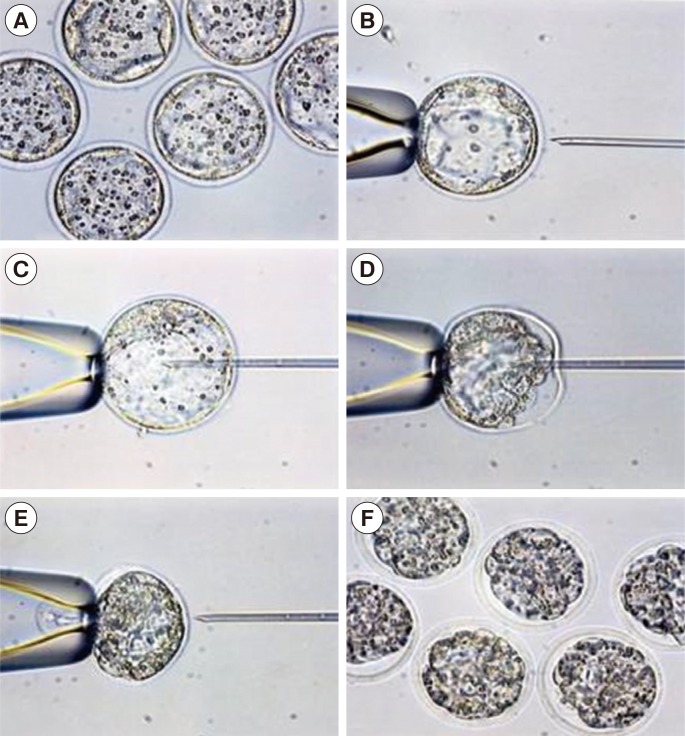
Figure┬Ā1
Stepwise photographs of artificial shrinkage (├Ś200). (A) Expanding mouse blastocyst before vitrification. (B) Retention of an expanding mouse blastocyst with a holding pipette. (C) Insertion of an intracytoplasmic sperm injection pipette inside the blastocoelic cavity. (D) Aspiration of blastocoelic fluid. (E) Removal of pipette after aspiration of blastocoele fluid. (F) Artificially shrunken mouse blastocysts.
Figure┬Ā2
(A-F) Photographs of the assisted hatching procedure of an expanding mouse blastocyst with micropipette after thawing (├Ś200).

-
METRICS

- Related articles in Clin Exp Reprod Med
-
New strategies for germ cell cryopreservation: Cryoinjury modulation2023 December;50(4)







