|
 |
- Search
| Clin Exp Reprod Med > Volume 40(2); 2013 > Article |
Abstract
To overcome the difficulty of controlling stem cell fate and function in applications to regenerative medicine, a number of alternative approaches have been made. Recent reports demonstrate that a non-cellular niche modulating the biophysical microenvironment with chemical factors can support stem cell self-renewal. In our previous studies, early establishment was executed to optimize biophysical factors and it was subsequently found that the microgeometry of the extracellular matrix made huge differences in stem cell behavior and phenotype. We review here a three-dimensional, non-cellular niche designed to support stem cell self-renewal. The characteristics of stem cells under the designed system are further discussed.
Self-renewal activity and the ability to differentiate into various functional cells are two major characteristics of stem cells [1]. Stem cells have thus been considered among the therapeutic biomaterials of use in regenerative medicine, which can restore the function of damaged cells, tissues, or organs. Before clinical application, however, a number of difficulties in manipulating stem cells should be overcome, and a variety of alternative technologies have been suggested to control stem cell fate and function.
Recent studies have demonstrated that conventional stem cell technologies do not meet the minimal requirements to precisely regulate stem cell function [2]. To overcome this limitation, several scientists have taken an interest in regulating stem cells under a specific microenvironment, which uses artificial extracellular matrices (ECMs) to control the stem cell self-renewal and differentiation activity [3]. The use of artificial ECMs for creating three-dimensional (3D) microenvironments would yield several benefits, mimicking the in vivo microenvironment of the undifferentiated or differentiated period at both the cell and tissue levels [4]. Biophysical microenvironments, including the stiffness of the substrate, nanotopography of the adhesion surface, microgeometric forces, and extracellular forces, must be constructed prior to the use of this 3D system, and the influence of stem cell fate in vitro through this artificial microenvironment can efficiently prevent the direct genetic manipulation of stem cells, which would otherwise limit the feasibility of clinical application [5-8]. Having access to artificial ECMs thus accelerates niche-related studies on manipulating stem cell self-renewal and differentiation; the data obtained from 3D cultures can be comparable to those obtained from conventional two-dimensional (2D) cultures.
As an initial step, most studies have employed embryonic stem cells (ESCs) in both experimental animals and humans. However, data from the culture of stem cells under conventional 2D systems provide a wealth of information that should assist in the development of innovative 3D culture systems. Therefore, careful consideration of stem cell culture in 2D systems is a prerequisite for developing 3D culture systems.
ESCs have generally been co-cultured with feeder cells such as xenogenic embryonic fibroblasts to maintain self-renewal activity. The feeder cells used for stem cell culture are able to supply growth factors, cytokines, and other extracellular matrix components such as leukemia inhibitory factor (LIF), activin, Wnt, bone morphogenetic proteins (BMPs), insulin-like growth factor (IGF), laminin, and vitronectin for maintaining the undifferentiated state of ESCs [9,10], which can create a suitable 2D environment. When applying these 2D systems to human ESCs, animal-derived feeder cells can cause unexpected disadvantages such as uncertain data outcomes and xenotransmission of unknown pathogens [11]. To avoid these handicaps, studies on the development of feeder-free cultures and defined culture systems have been strongly encouraged [12].
Since the initial studies of Richards et al. [13], human feeder cells such as neonatal foreskin fibroblasts [14], fetal muscle, fetal skin, adult fallopian tube epithelial cells [13], adult muscle, adult skin [15], marrow-derived stromal cells [16], amniotic fluid fibroblasts [17], placenta-derived fibroblasts [18], and human ESC (hESC)-derived fibroblasts [19] have been employed to provide a suitable cellular niche for hESCs without the use of xenogenic cells. A positive outcome of the replacement was reported [15], although batch differences in feeder cell-based culture methods is considered another uncertainty that impedes the establishment of stable culture conditions. To further develop defined stem cell culture systems, the use of non-cellular niches can subsequently be considered.
An initial attempt has been made to employ non-cellular niches for the development of defined ESC culture systems, and artificial ECMs were thus employed. Instead of feeder cells, Xu et al. [12] used Matrigel-coated dishes. ESCs were successfully cultured with the use of fibroblast-conditioned medium, to which co-culture technology was applied for the culture of other stem cell lines [20]. Each medium conditioned with different cells had a different capacity to maintain ESC self-renewal [21]. However, some showed negative results in attempts to support the long-term culture of ESCs, which demonstrates their unsuitability as a standardized culture regime [22]. Such a limitation directly encourages the development of a defined culture medium and the refinement of ESCs for suitable ESC culture methods.
Amit et al. [23] first reported the successful use of knockout serum for ESC culture as a replacement for bovine serum, although this semi-defined serum replacement contains xenogenic or undefined substances such as bovine serum albumin [24]. Subsequently, a culture medium containing human-originated recombinant proteins was developed (X-VIVO 10 medium). As suitable supplements for hESC culture, basic fibroblast growth factor (b-FGF), stem cell factor (SCF), recombinant human FMS-like tyrosine kinase 3 ligand (rhFlt3L), and LIF [25] were recommended. TeSR1 using recombinant proteins and purified material from humans was also suggested as a culture supplement for hESC culture [26]. Discovery of the alternative material Matrigel was developed and the single use of laminin, one of the Matrigel components, was successful for the culture of undifferentiated hESCs [16]. It has also been reported that laminin isoforms 111, 332, and 511, vitronectin, and E-cadherin support the adhesion and proliferation of hESCs [27-29].
Among 3D scaffolds being constructed with various biocompatible biomaterials, hydrogel-based ECMs have been employed for the culture of stem cells. The hydrogel is a cell-friendly, 3D macromolecule platform formed by the crosslinking of hydrophilic polymers, which can absorb H2O molecules to over five hundred times its own weight. In response to the salt concentration, pH, temperature, and crosslinking method, the chemical structure of the hydrogel can change in unlimited ways, so many biomedical [30] and pharmaceutical [31] applications may be possible, such as drug delivery [30], artificial tissue [32], membrane fabrication [33], and matrix construction [34].
To date, natural material-based hydrogel systems have been developed. Hydrogel-based, protein-like materials such as Matrigel [35,36], collagen [37], fibrin [38], and silk [39,40], polysaccharide-like materials such as hyaluronic acid [41,42], alginate [43,44], dextran [30], chitosan [45,46], and agarose [47], and DNA [48] have been used. In most of these cases, however, difficulties with manipulating their mechanical properties, the degradation rate, and the reproducibility occurred due to their undefined protein composition, which limited the modification of binding domains or sites in cellular activity and immune responses. To overcome this limitation, a hydrogel-based, synthetic (chemically defined) material has subsequently been suggested, which has a number of advantages including easy manipulation of mechanical properties, degradation, shape, and reproducibility. Many biological processes can be regulated by the use of synthetic hydrogel components, which further makes it possible to develop transplantable biomaterials. Hydrogels polymerized with peptide [49] or poly (lactic-co-glycolic acid) (PLGA) [50] have subsequently been developed, and recently, the use of poly (ethylene glycol) (PEG)-based hydrogel systems [51] has drawn attention. This newly developed hydrogel complex yields many benefits because of its conjugating activity, and it is difficult to induce bio-degradation into the monomers by natural metabolites, or cosmetic and medical substances. This biocompatible material was approved by the FDA in United States.
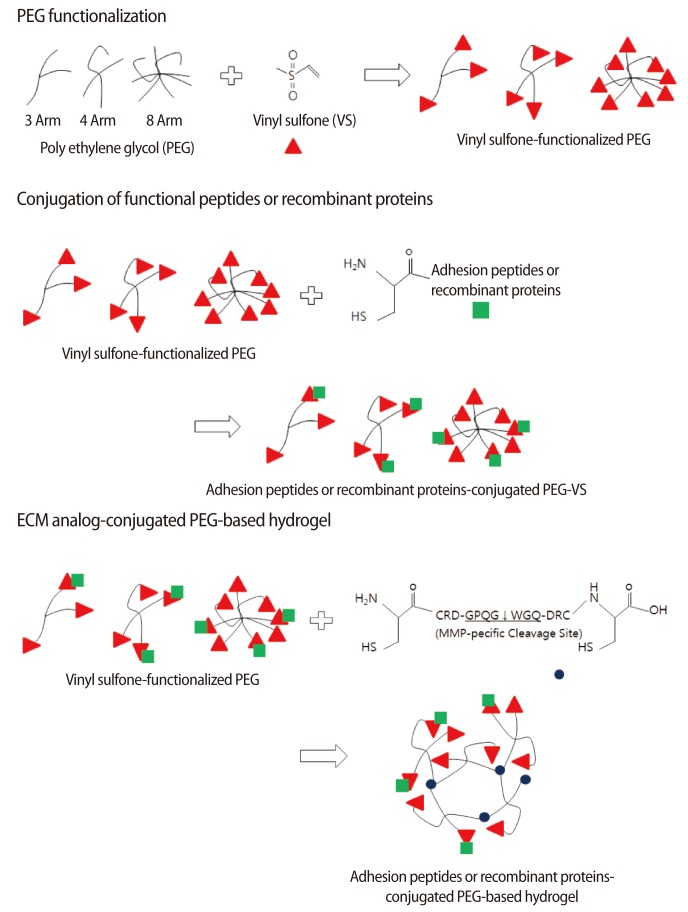
To create a cell-friendly microenvironment, a hydrogel polymerized by polyethylene glycol (PEG) has been suggested. In nature, the polymerization of diverse functional groups and functionalized PEG was conducted under cell-unfriendly conditions, which resulted in cellular toxicity. To avoid this insufficiency, a remodeled 3D scaffold system has been suggested (Figure 1). In this system, vinyl sulfone (VS)-functionalized PEG, which contains dicystein-containing peptide having matrix metalloproteinase (MMP)-specific cleavage sites (hereafter referred to as a combining region of the matrices with crosslinker), is employed. Therefore, conjugation of the sulfhydryl (SH) group of cystein with the VS group of PEG is conducted spontaneously through a Michael-type addition reaction in a cell-friendly environment [52]. Based on these previous achievements, employing VS-functionalized hydrogel is now being employed in the design of artificial niches for regulating stem-cell function and activity.
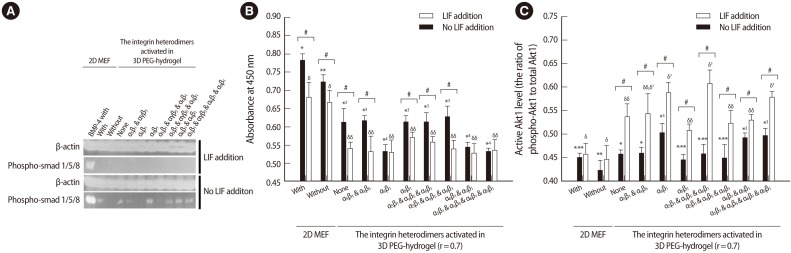
Using PEG-VS and a crosslinker, an ECM analog of an acellular niche has been developed and the microenvironmental influence of this 3D niche on self-renewal was evaluated in mouse ESCs (mESCs) [53]. The initial study evaluated the transcriptional, translational, and functional activity of integrin subunits and subsequently identified the expression of integrin ╬▒5╬▓1, ╬▒V╬▓5, ╬▒6╬▓1, and ╬▒9╬▓1 in the membranes of undifferentiated mESCs. These integrin heterodimers play important roles in ESC self-renewal and select RGDSP, TTSWSQ, and AEIDGIEL peptide motifs to co-activate integrin ╬▒5╬▓1, ╬▒V╬▓5, ╬▒6╬▓1, and ╬▒9╬▓1 (Figure 2). In next study, the effectiveness of PEG-based hydrogels of different mechanical properties, which were modulated by the ratio of (VS) and (SH) concentration (hereafter referred to as the stoichiometric ratio), was evaluated. The mechanical properties of the hydrogel with a 0.7 stoichiometric ratio significantly stimulated mESC self-renewal (Figure 3). Combinatorial conjugation of the optimized ECM analogs with selected motifs showed that four integrin heterodimers promoted self-renewal of mESCs. Subsequently, strong expression of stemness-related genes, which was similar to the expression under a conventional 2D cellular niche, was detected. Self-renewal of mESCs under the designed 3D acellular biomimetic niche was successfully maintained without stimulation of LIF signaling [54], and Stat3 activation by exogenous LIF is no longer the rate-limiting factor of stem cell self-renewal under a designated niche. Instead, Akt1/Smad signaling was significantly activated in the mESCs maintained under the 3D microenvironment (Figure 4). These data clearly indicate that a different pathway of ESC self-renewal is activated to adapt to a different microenvironment. Although significant changes in morphological properties were detected, this signal shift under a different niche does not cause any initial change in ESC self-renewal activity.
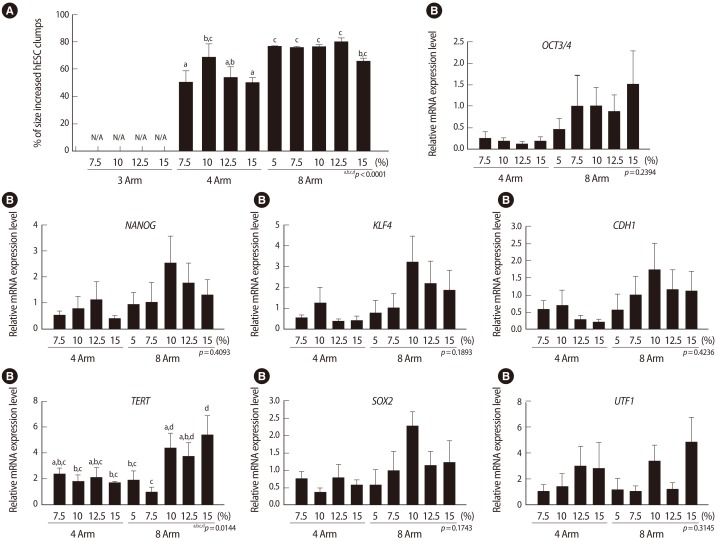
In the case of hESCs, a 3D acellular niche showed the potential to support hESC self-renewal without ECM analogs [54]. The optimal properties of 3D PEG-based hydrogel for the H9 hESC line was examined and the mechanical properties of the hydrogel were modified by the VS-functionalized PEG multiarm number and PEG concentration. It was found that hESC self-renewal and stemness were the highest after encapsulation in 8-multiarm PEG hydrogel consisting of 10% (wt/v) PEG (Figure 5). This optimized PEG-hydrogel was successfully applied to the 3D culture of H1 and Novo hESC lines. The 3D scaffold reduced the difference in the proliferative activity among three hESC lines evaluated. However, it does not seem that this optimized condition is sufficient for long-term maintenance of hESCs.
The possibility of maintaining stem cell self-renewal was found under a defined 3D niche, which is different from a conventional 2D niche. The PEG-based hydrogel using PEG-VS and crosslinkers containing both SH group and MMP-specific cleavage sites greatly contributes to establishing a cell-friendly 3D non-cellular niche system, which is capable of constructing diverse niches by mimicking different biomechanics and patterning after diverse ECM analogs. Probably, the suggested, hydrogel-nased system can be useful for creating cell-friendly environment in vitro. However, further trials to improve this 3D non-cellular niche for ESC self-renewal should be conducted for field and clinical application. There have been only a few applications using any 3D culture system, but more extensive studies about the characterization of stem cell niches in vivo. Attempts to synthesize ECM analogs or recombinant proteins, or to discover conjugating peptides will suggest a variety of alternative technologies for stem cell manipulation.
References
1. Ding S, Schultz PG. A role for chemistry in stem cell biology. Nat Biotechnol 2004;22:833-840.PMID: 15229546.


2. Nava MM, Raimondi MT, Pietrabissa R. Controlling self-renewal and differentiation of stem cells via mechanical cues. J Biomed Biotechnol 2012;2012:797410PMID: 23091358.



3. Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 2009;5:17-26.PMID: 19570510.



4. Burdick JA, Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng Part A 2009;15:205-219.PMID: 18694293.


5. Castillo AB, Jacobs CR. Mesenchymal stem cell mechanobiology. Curr Osteoporos Rep 2010;8:98-104.PMID: 20425617.


6. Hayashi Y, Furue MK, Okamoto T, Ohnuma K, Myoishi Y, Fukuhara Y, et al. Integrins regulate mouse embryonic stem cell self-renewal. Stem Cells 2007;25:3005-3015.PMID: 17717067.


7. Reilly GC, Engler AJ. Intrinsic extracellular matrix properties regulate stem cell differentiation. J Biomech 2010;43:55-62.PMID: 19800626.


8. Watanabe S, Umehara H, Murayama K, Okabe M, Kimura T, Nakano T. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene 2006;25:2697-2707.PMID: 16407845.


9. Alitalo K, Kuismanen E, Myllyla R, Kiistala U, Asko-Seljavaara S, Vaheri A. Extracellular matrix proteins of human epidermal keratinocytes and feeder 3T3 cells. J Cell Biol 1982;94:497-505.PMID: 6182145.



10. Talbot NC, Sparks WO, Powell AM, Kahl S, Caperna TJ. Quantitative and semiquantitative immunoassay of growth factors and cytokines in the conditioned medium of STO and CF-1 mouse feeder cells. In Vitro Cell Dev Biol Anim 2012;48:1-11.PMID: 22179674.


11. Lei T, Jacob S, Ajil-Zaraa I, Dubuisson JB, Irion O, Jaconi M, et al. Xeno-free derivation and culture of human embryonic stem cells: current status, problems and challenges. Cell Res 2007;17:682-688.PMID: 17667917.


12. Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol 2001;19:971-974.PMID: 11581665.


13. Richards M, Fong CY, Chan WK, Wong PC, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol 2002;20:933-936.PMID: 12161760.


14. Hovatta O, Mikkola M, Gertow K, Stromberg AM, Inzunza J, Hreinsson J, et al. A culture system using human foreskin fibroblasts as feeder cells allows production of human embryonic stem cells. Hum Reprod 2003;18:1404-1409.PMID: 12832363.


15. Richards M, Tan S, Fong CY, Biswas A, Chan WK, Bongso A. Comparative evaluation of various human feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells 2003;21:546-556.PMID: 12968109.


16. Cheng L, Hammond H, Ye Z, Zhan X, Dravid G. Human adult marrow cells support prolonged expansion of human embryonic stem cells in culture. Stem Cells 2003;21:131-142.PMID: 12634409.


17. Kim HS, Seol HW, Ahn HJ, Oh SK, Ku SY, Kim SH, et al. Human amniotic fluid cells support expansion culture of human embryonic stem cells. Korean J Fertil Steril 2004;31:261-272.
18. Genbacev O, Krtolica A, Zdravkovic T, Brunette E, Powell S, Nath A, et al. Serum-free derivation of human embryonic stem cell lines on human placental fibroblast feeders. Fertil Steril 2005;83:1517-1529.PMID: 15866593.


19. Wang Q, Fang ZF, Jin F, Lu Y, Gai H, Sheng HZ. Derivation and growing human embryonic stem cells on feeders derived from themselves. Stem Cells 2005;23:1221-1227.PMID: 15955827.


20. Mallon BS, Park KY, Chen KG, Hamilton RS, McKay RD. Toward xeno-free culture of human embryonic stem cells. Int J Biochem Cell Biol 2006;38:1063-1075.PMID: 16469522.



21. Tsai ZY, Singh S, Yu SL, Chou CH, Li SS. A feeder-free culture using autogeneic conditioned medium for undifferentiated growth of human embryonic stem cells: comparative expression profiles of mRNAs, microRNAs and proteins among different feeders and conditioned media. BMC Cell Biol 2010;11:76PMID: 20937144.



22. Amit M, Itskovitz-Eldor J. Feeder-free culture of human embryonic stem cells. Methods Enzymol 2006;420:37-49.PMID: 17161692.


23. Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol 2000;227:271-278.PMID: 11071754.


24. Hewitt ZA, Amps KJ, Moore HD. Derivation of GMP raw materials for use in regenerative medicine: hESC-based therapies, progress toward clinical application. Clin Pharmacol Ther 2007;82:448-452.PMID: 17687270.


25. Li Y, Powell S, Brunette E, Lebkowski J, Mandalam R. Expansion of human embryonic stem cells in defined serum-free medium devoid of animal-derived products. Biotechnol Bioeng 2005;91:688-698.PMID: 15971228.


26. Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol 2006;24:185-187.PMID: 16388305.


27. Braam SR, Zeinstra L, Litjens S, Ward-van Oostwaard D, van den Brink S, van Laake L, et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells 2008;26:2257-2265.PMID: 18599809.


28. Miyazaki T, Futaki S, Hasegawa K, Kawasaki M, Sanzen N, Hayashi M, et al. Recombinant human laminin isoforms can support the undifferentiated growth of human embryonic stem cells. Biochem Biophys Res Commun 2008;375:27-32.PMID: 18675790.


29. Xu Y, Zhu X, Hahm HS, Wei W, Hao E, Hayek A, et al. Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc Natl Acad Sci U S A 2010;107:8129-8134.PMID: 20406903.



30. Sun G, Mao JJ. Engineering dextran-based scaffolds for drug delivery and tissue repair. Nanomedicine (Lond) 2012;7:1771-1784.PMID: 23210716.



31. Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm 2000;50:27-46.PMID: 10840191.


32. Gong Y, Su K, Lau TT, Zhou R, Wang DA. Microcavitary hydrogel-mediating phase transfer cell culture for cartilage tissue engineering. Tissue Eng Part A 2010;16:3611-3622.PMID: 20666616.



33. Choi E, Jun I, Chang HK, Park KM, Shin H, Park KD, et al. Quantitatively controlled in situ formation of hydrogel membranes in microchannels for generation of stable chemical gradients. Lab Chip 2012;12:302-308.PMID: 22108911.


34. Chen H, Xiao L, Du D, Mou D, Xu H, Yang X. A facile construction strategy of stable lipid nanoparticles for drug delivery using a hydrogel-thickened microemulsion system. Nanotechnology 2010;21:015101PMID: 19946154.


35. Kang BJ, Ryu HH, Park SS, Kim Y, Woo HM, Kim WH, et al. Effect of matrigel on the osteogenic potential of canine adipose tissue-derived mesenchymal stem cells. J Vet Med Sci 2012;74:827-836.PMID: 22313966.


36. Rao SS, Bentil S, DeJesus J, Larison J, Hissong A, Dupaix R, et al. Inherent interfacial mechanical gradients in 3D hydrogels influence tumor cell behaviors. PLoS One 2012;7:e35852PMID: 22558241.



38. Haugh MG, Thorpe SD, Vinardell T, Buckley CT, Kelly DJ. The application of plastic compression to modulate fibrin hydrogel mechanical properties. J Mech Behav Biomed Mater 2012;16:66-72.PMID: 23149099.


39. Talukdar S, Mandal M, Hutmacher DW, Russell PJ, Soekmadji C, Kundu SC. Engineered silk fibroin protein 3D matrices for in vitro tumor model. Biomaterials 2011;32:2149-2159.PMID: 21167597.


40. Wang X, Sun L, Maffini MV, Soto A, Sonnenschein C, Kaplan DL. A complex 3D human tissue culture system based on mammary stromal cells and silk scaffolds for modeling breast morphogenesis and function. Biomaterials 2010;31:3920-3929.PMID: 20185172.



41. Gurski LA, Jha AK, Zhang C, Jia X, Farach-Carson MC. Hyaluronic acid-based hydrogels as 3D matrices for in vitro evaluation of chemotherapeutic drugs using poorly adherent prostate cancer cells. Biomaterials 2009;30:6076-6085.PMID: 19695694.



42. Xu X, Gurski LA, Zhang C, Harrington DA, Farach-Carson MC, Jia X. Recreating the tumor microenvironment in a bilayer, hyaluronic acid hydrogel construct for the growth of prostate cancer spheroids. Biomaterials 2012;33:9049-9060.PMID: 22999468.



43. Huang X, Zhang X, Wang X, Wang C, Tang B. Microenvironment of alginate-based microcapsules for cell culture and tissue engineering. J Biosci Bioeng 2012;114:1-8.PMID: 22561878.


44. Sidhu K, Kim J, Chayosumrit M, Dean S, Sachdev P. Alginate microcapsule as a 3D platform for propagation and differentiation of human embryonic stem cells (hESC) to different lineages. J Vis Exp 2012;(61): e3608.

45. Zhang Y, Choi SW, Xia Y. Modifying the pores of an inverse opal scaffold with chitosan microstructures for truly three-dimensional cell culture. Macromol Rapid Commun 2012;33:296-301.PMID: 22231861.


46. Zhu JH, Wang XW, Ng S, Quek CH, Ho HT, Lao XJ, et al. Encapsulating live cells with water-soluble chitosan in physiological conditions. J Biotechnol 2005;117:355-365.PMID: 15925718.


47. Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 1982;30:215-224.PMID: 7127471.


48. Um SH, Lee JB, Park N, Kwon SY, Umbach CC, Luo D. Enzyme-catalysed assembly of DNA hydrogel. Nat Mater 2006;5:797-801.PMID: 16998469.


49. Gauthier MA, Klok HA. Peptide/protein-polymer conjugates: synthetic strategies and design concepts. Chem Commun (Camb) 2008;2591-2611.PMID: 18535687.


50. Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci U S A 2003;100:12741-12746.PMID: 14561891.



51. Dickinson LE, Kusuma S, Gerecht S. Reconstructing the differentiation niche of embryonic stem cells using biomaterials. Macromol Biosci 2011;11:36-49.PMID: 20967797.


52. Lee ST, Yun JI, Jo YS, Mochizuki M, van der Vlies AJ, Kontos S, et al. Engineering integrin signaling for promoting embryonic stem cell self-renewal in a precisely defined niche. Biomaterials 2010;31:1219-1226.PMID: 19926127.


53. Lee ST, Yun JI, van der Vlies AJ, Kontos S, Jang M, Gong SP, et al. Long-term maintenance of mouse embryonic stem cell pluripotency by manipulating integrin signaling within 3D scaffolds without active Stat3. Biomaterials 2012;33:8934-8942.PMID: 22998814.


54. Jang M, Lee ST, Kim JW, Yang JH, Yoon JK, Park JC, et al. A feeder-free, defined three-dimensional polyethylene glycol-based extracellular matrix niche for culture of human embryonic stem cells. Biomaterials 2013;34:3571-3580.PMID: 23422594.


Figure┬Ā1
Schematic presentation of polyethylene glycol (PEG)-based hydrogel preparation. Mono-functional peptides or recombinant proteins containing an extracellular matrice (ECM) analog for cell signaling or adhesion are conjugated with multi-armed vinyl sulfone (VS)-functionalized PEG. Then, the dicystein-containing peptide, which has sequences that respond to cell-secreted enzymes like matrix metalloproteinase (MMP), is used for crosslinking. These PEG-based hydrogels lead to the formation of an elastic gel scaffold, providing specific extracellular signaling to cells, and the degradation to soluble PEG-monomers in the exposure of MMP by cleavage of the crosslinking peptides.
Figure┬Ā2
Binding of integrin heterodimers with functional units of peptides of sequence RGDSP, AEIDGIEL, and TTSWSQ in surface membrane of mESCs. (A-C) Integrin ╬▒5╬▓1 and ╬▒V╬▓5 can be activated with RGDSP, a functional ligand of the integrins, in the mESCs. The binding activity of mESCs to RGDSP was significantly inhibited after being treated with anti-integrin ╬▒5 antibody, regardless of the treatment with anti-integrin ╬▒V antibody. However, combined treatment of both antibodies yielded the lowest attachment of ESCs to the RGDSP peptide. (D, E) Integrin ╬▒9╬▓1 activity was promoted by binding of AEIDGIEL with mESCs. Anti-integrin ╬▒9╬▓1 antibody significantly inhibited the binding of the mESCs with AEIDGIEL peptide. (F) TTSWSQ-induced, activity of integrin ╬▒6╬▓1 activity in a 3D culture using PEG-based scaffolds. The mESCs without exposure to anti-integrin ╬▒6 antibody significantly increased the transcription level of all genes tested after the TTSWSQ conjugation, while there was no change in the gene expression after the exposure of the antibody-treated ESCs to TTSWSQ. All data shown are represented as means┬▒SD of three independent experiments. *,**p<0.05. (From Lee et al. [52]. Biomaterials 2010;31:1219-26, with permission from Elsevier).

Figure┬Ā3
The effects of hydrogel-internal mechanical properties on the physiology and self-renewal of mouse embryonic stem cells (mESCs). In the presence of leukemia inhibitory factor (LIF), the mESCs were cultured for 5 day under varied mechanical conditions derived from different stoichiometric ratios (r) of the PEG-based scaffold (A). A significant increase in the size of mESC colonies (B) was observed with the use of polyethylene glycol (PEG)-hydrogels having a 0.6 and 1.1 r-value (soft gels), while no significant difference in mitochondrial metabolism (C) or alkaline phosphatase (AP) activity (D) was detected after changing the elasticity of the PEG hydrogel. The PEG hydrogel with a 0.7 r-value showed the highest expression of most stemness-related genes (E). Data on the size of the colonies (B) are represented by box and whisker plots: the box indicates the range between the 25th and 75th percentiles, and the straight and dotted lines in the box show the median and mean values, respectively. The whiskers represent the distribution of values, and the dots show the minimal and maximal numbers. Moreover, in the data on the mechanical properties, mitochondrial metabolic and AP activity are presented as means┬▒SD of three or more independent experiments. Different colors in Figure (E) indicate significant differences (p<0.05). *p<0.05. (From Lee et al. [52]. Biomaterials 2010;31:1219-26, with permission from Elsevier).

Figure┬Ā4
Translational regulation of Smad 1/5/8 and Akt1 signal molecules in mESCs cultured in 2D with ('With') or without ('Without') MEFs and within 3D PEG scaffolds with (as indicated) or without ('None') integrin activation in the absence of LIF. In the absence of LIF, strong activation of both Smad 1/5/8 and Akt1 kinase resulted from stimulation of integrins ╬▒5╬▓1, ╬▒v╬▓5, ╬▒6╬▓1, and ╬▒9╬▓1 (A, C), and the 3D culture of the ESCs induced activation of both Smad 1/5/8 (A) and Akt1 (C) but concomitantly inhibited expression of total Akt1, regardless of leukemia inhibitory factor (LIF) supplementation (B). Complete inhibition of Smad 1/5/8 activation was caused by LIF supplementation to all the microenvironments (A) and the activity of Akt1 kinase in the absence of LIF was significantly stronger than that in the presence of LIF, regardless of the type of integrin stimulation (C). Western blot, n=5; ELISA, n=5. Error bars represent SD. *-****p<0.05. ╬┤-╬┤╬┤╬┤p<0.05. #p<0.05. (From Lee et al. [53]. Biomaterials 2012;33:8934-42, with permission from Elsevier).
Figure┬Ā5
Optimization of mechanical stiffness of a polyethylene glycol (PEG)-based hydrogel to increase cell proliferation and the expression of stemness-related genes in H9 human embryonic stem cells (hESCs). (A) Regardless of gel concentration, large H9 ESC clumps were found in 4-arm and 8-arm hydrogels. The H9 hESCs cultured in 3-arm hydrogel, which is softer than 4-arm and 8-arm hydrogels, were not maintained. (B) No significant difference except for in TERT was detected between the 4-arm and 8-arm hydrogel groups, while TERT expression was higher in the H9 hESCs cultured in 10% to-15% 8-arm hydrogel than those cultured in the hydrogel of other compositions. All data shown are mean┬▒SD from the values of three replicates. N/A, not analyzed. (From Jang et al. [54]. Biomaterials 2013;34:3571-80, with permission from Elsevier).