 |
 |
- Search
| Clin Exp Reprod Med > Volume 47(2); 2020 > Article |
|
Abstract
Objective
To investigate whether the degree of post-warming embryo or blastocyst development is associated with clinical pregnancy in vitrified embryo or blastocyst transfer cycles.
Methods
Ninety-six vitrified cleavage-stage embryos and 58 vitrified blastocyst transfer cycles were selected. All transfer cycles were performed from February 2011 to March 2019, and all vitrified embryos or blastocysts were warmed from 4 PM to 6 PM and then transferred the next morning from 9 AM to 10 AM. The scores of the cleavage-stage embryos and blastocysts were assessed at warming and at transfer using the modified Steer method and the Gardner method, respectively. The mean embryo or blastocyst score, score of the single top-quality embryo or blastocyst, and the difference in the score between warming and transfer were compared between nonpregnant and pregnant women.
Results
In the cleavage-stage embryo transfer cycles, both the top-quality embryo score at transfer and the difference in the score between warming and transfer were significantly associated with clinical pregnancy. A top-quality embryo score at transfer of ≥60.0 (area under the curve [AUC], 0.673; 95% confidence interval [CI], 0.531–0.815) and a difference in the score between warming and transfer of ≥23.0 (AUC, 0.675; 95% CI, 0.514–0.835) were significant predictors of clinical pregnancy. In blastocyst transfer cycles, the top-quality blastocyst score at transfer was the only significant factor associated with clinical pregnancy. A top-quality blastocyst score at transfer of ≥38.3 was a significant predictor of clinical pregnancy (AUC, 0.666; 95% CI, 0.525–0.807).
Conclusion
The top-quality embryo score at transfer and the degree of post-warming embryo development were associated with clinical pregnancy in vitrified cleavage-stage embryo transfer cycles. In vitrified blastocyst transfer cycles, the top-quality blastocyst score at transfer was the only significant factor affecting clinical pregnancy.
Embryo cryopreservation is usually performed when surplus embryos are obtained or ovarian hyperstimulation syndrome is strongly suspected during in vitro fertilization (IVF) cycles [1-3]. The later use of cryopreserved embryos would enhance the cumulative pregnancy rate per patient [4]. In cryopreserved embryo transfer cycles, a higher implantation rate has been observed in women with a successful previous fresh embryo transfer cycle, age < 40 years, and non-tubal factor infertility [5]. The delivery rate after frozen cleavage-stage embryo transfer cycles was found to be dependent on both the woman’s age and the quality of embryos transferred [6].
In cryopreserved blastocyst transfer cycles, significant correlations were observed between blastocyst quality or women’s age and the clinical pregnancy or delivery rate [7]. Honnma et al. [8] found that pre-freezing trophectoderm morphology significantly affected the ongoing pregnancy and miscarriage rates in frozen single blastocyst transfer cycles; in contrast, Zhang et al. [9] observed that the clinical pregnancy rate was affected by pre-freezing inner cell mass morphology. In transfer cycles involving euploid blastocysts, pre-freezing inner cell mass morphology affected implantation and ongoing pregnancy rates [10]. In a study of single frozen blastocyst transfer cycles, Hur et al. [11] found that the clinical pregnancy/implantation rate depended on whether the transferred blastocyst was hatched, but was not associated with the blastocyst grade. In cryopreserved embryo or blastocyst transfer cycles, embryos or blastocysts are usually thawed or warmed in the evening of the day before transfer; thus, embryos or blastocysts usually develop spontaneously until transfer.
In an earlier report, transfers of frozen cleavage-stage embryos that continue to cleave in vitro after thawing were found to result in a higher delivery rate than mixed transfers of embryos with and without further cleavage [12]. In that report, transfers of only embryos without further cleavage did not lead to any deliveries. Similarly, transferring at least one embryo with further cleavage during 24 hours post-thawing showed a significantly higher implantation/clinical pregnancy rate than transfers of embryos that did not undergo further cleavage [13].
Van Landuyt et al. [14] reported that vitrified day-3 embryos developed better overnight than slowly frozen embryos. Moreover, they found that there was no effect of the number of cells lost or the cryopreservation method on the implantation potential of the embryo if it continued to cleave after thawing or warming. Fernandez Gallardo et al. [15] reported that the implantation rate of post-warming embryos was determined by the occurrence of mitosis resumption, the number of cells lost, and the specific number of blastomeres, but not by fragmentation, blastomere symmetry, or volume change. Ahlstrom et al. [16] found that post-thawing degree of blastocoele reexpansion was the most significant predictor of live birth in frozen blastocyst transfer cycles.
The majority of the studies indicated that further cleavage (i.e., mitosis resumption) post-thawing or post-warming was an important factor for predicting cycle outcomes. To date, no study has investigated the clinical pregnancy rate based on the degree of post-warming embryo or blastocyst development. In the present study, we evaluated whether the degree of embryo or blastocyst development during overnight culture influenced the clinical pregnancy rate in vitrified embryo or blastocyst transfer cycles.
This retrospective study included 96 vitrified cleavage-stage embryo transfer cycles and 58 vitrified blastocyst transfer cycles. We included nonselected frozen embryo transfer cycles performed at Seoul National University Bundang Hospital from February 2011 to March 2019, if at least one embryo survived after warming and was then transferred into the uterus. We excluded cases where no embryos or blastocysts survived after warming or if transferrable embryos were not obtained after overnight culture. We obtained Institutional Review Board approval of Seoul National University Bundang Hospital for the use of patients’ medical records (IRB No. B-1901/516-107).
All embryos or blastocysts from previous fresh cycles of each patient were vitrified with equilibrium solution (ES) and vitrification solution (VS) using a CryoTop device (Kitazato, Tokyo, Japan). For the vitrification process, embryos were suspended in ES containing 7.5% ethylene glycol (EG; Sigma-Aldrich, St. Louis, MO, USA) and 7.5% dimethyl sulfoxide (DMSO; Sigma-Aldrich) in basic medium (Global for Fertilization, LifeGlobal, Guilford, CT, USA) for 5 minutes at room temperature (RT), and transferred to VS containing 15% EG, 15% DMSO, and 0.5 mol/L sucrose (Sigma-Aldrich) in basic medium for 45–60 seconds at RT. The embryos or blastocysts were loaded into a CryoTop and then immediately plunged into liquid nitrogen for storage.
In the vitrified cleavage-stage embryo transfer cycles, the mean age of the women and their husbands was 36.4 ± 4.5 and 39.5 ± 5.0 years, respectively. The indications for IVF were female factor infertility (57 cycles), male (21 cycles), unexplained infertility (15 cycles), and combined factor infertility (3 cycles). In the vitrified blastocyst transfer cycles, the mean age of the women and their husbands was 33.8 ± 3.5 and 37.1 ± 4.5 years, respectively. The indications for IVF were female factor infertility (36 cycles), male factor infertility (7 cycles), and unexplained infertility (15 cycles). In five of the 58 blastocyst transfer cycles, the blastocysts were obtained on day 6.
Endometrial preparation was performed following a similar method to that described in our previous report [17]. In natural cycles, no additional medication was used for endometrial preparation, and only recombinant human chorionic gonadotropin (Ovidrel; Merck-Serono, Darmstadt, Germany) was used for ovulation triggering. In the hormone replacement cycle, estradiol valerate (Progynova; Bayer, Leverkusen, Germany) was initiated on the 3rd or 4th day of the menstrual cycle. When the endometrial thickness was > 7 mm, an intramuscular injection of 50 mg of progesterone (Genefer; Watson Laboratories Inc., Salt Lake City, UT, USA or Taiyu progesterone; Taiyu Chemical & Pharmaceutical, Taiwan), micronized vaginal progesterone (Lutinus; Ferring, Kiel, Germany), or a vaginal progesterone gel (Crinone; Merck-Serono) was initiated.
When the vitrified embryos or blastocysts were warmed, the CryoTop was immersed directly in 37°C warming solution (containing 1.0 mol/L sucrose in basic medium) for 1 minute. The embryos were then immediately transferred to dilution solutions (0.5 mol/L and 0.25 mol/L sucrose in basic medium) serially and incubated at RT for 3 minutes, respectively, and then washed twice with basic medium. The warmed embryos were transferred to the culture medium (Sydney IVF Medium; Cook Medical, Eight Mile Plains, Australia) and cultured overnight until transfer at 37°C and maintained with 5% CO2 in humidified air. In all cycles, embryos or blastocysts were warmed from 4 PM to 6 PM, and then transferred the next morning from 9 AM to 10 AM. Clinical pregnancy was defined as the identification of at least one gestational sac in the uterine cavity.
The score for cleavage-stage embryos was assessed by the Steer method [18]. After multiplying the cell number by each grading score (A = 4, B = 3, C = 2, D = 1), the scores were summed. The mean embryo score was obtained as the summed embryo score divided by the number of embryos transferred. Compactions were considered to be a super-staged (5 points) 8-cell embryos, so a score of 40 (8 × 5) was assigned. A morula was considered to be an advanced-stage (6 points) 12-cell embryo, so a score of 72 (12 × 6) was assigned. If an embryo developed to the blastocyst stage during overnight culture, it was scored as 160. The single embryo showing the best score was regarded as the top-quality embryo. The mean embryo score and the score of the top-quality embryo were assessed both at warming and transfer.
The score for blastocysts was assessed as the (development score) × (inner cell mass score) × (trophectoderm score) [19]. The development score was assigned as follows: early blastocyst = 1, middle expanding blastocyst =2, expanded or fully expanded blastocyst = 3.5, expanded blastocyst with partial hatching = 5, fully hatched = 6. The scores for the inner cell mass and trophectoderm were assigned separately based on their grade, as follows: A = 3, B = 2, C = 1. For example, an expanded blastocyst with partial hatching and grade BB was assigned a score of 20 (5 × 2 × 2). If two or more blastocysts were present, the mean blastocyst score was obtained as the summed score divided by the number of blastocysts transferred. The single blastocyst showing the best score was regarded as the top-quality blastocyst. The mean blastocyst score and the score of the top-quality blastocyst were assessed both at warming and transfer.
Parameters were compared between the nonpregnant and pregnant groups using the Mann-Whitney U-test or the chi-square test, as appropriate. The data are shown as median and 95% confidence interval (CI). A p-value < 0.05 was considered to indicate statistical significance. If a parameter was found to be statistically significant, a receiver operating characteristic curve was constructed to obtain a specific cut-off value. The area under the curve (AUC) with its 95% CI was obtained, and a 95% CI above 0.5 was considered as to indicate significance.
In total, 221 cleavage-stage embryos were warmed and all survived (survival rate, 100%). However, seven cleavage-stage embryos degenerated (i.e., the cell number decreased or the embryo was downgraded) after overnight culture, making the degeneration rate 3.2% per embryo. A total of 111 blastocysts were warmed and 105 survived (survival rate, 94.6%). However, seven blastocysts degenerated after overnight culture, making the degeneration rate 6.7% per blastocyst.
In vitrified cleavage-stage embryo transfer cycles, the clinical pregnancy rate was 16.7% (16/96). As shown in Table 1, basal characteristics, the method of endometrial preparation, the number of embryos transferred, the mean embryo score at warming, the difference in the score between warming and transfer, and the top-quality embryo score at warming were all similar between the nonpregnant and pregnant group. However, both the top-quality embryo score at transfer and the difference in the score between warming and transfer were significantly higher in the pregnant group (p= 0.025, p= 0.027, respectively). A top-quality embryo score at transfer of ≥ 60.0 (AUC, 0.673; 95% CI, 0.531–0.815) and a difference in the score between warming and transfer of ≥ 23.0 (AUC, 0.675; 95% CI, 0.514–0.835) were significant predictors of clinical pregnancy (Table 2, Figures 1 and 2).
In vitrified blastocyst transfer cycles, the clinical pregnancy rate was 37.9% (22/58). As shown in Table 3, basal characteristics, the method of endometrial preparation, the number of blastocysts transferred, the mean blastocyst score either at warming or transfer, the difference in the score between warming and transfer, the top-quality blastocyst score at warming, and the difference in the score of top-quality embryo between warming and transfer were all similar between the nonpregnant and pregnant groups. However, the top-quality blastocyst score at transfer was significantly higher in the pregnant group (p= 0.031). A top-quality blastocyst score at transfer of ≥ 38.3 was a significant predictor of clinical pregnancy (AUC, 0.666; 95% CI, 0.525–0.807) (Table 4, Figure 3).
In the present study, two factors (the top-quality embryo score at transfer and the degree of post-warming embryo development) were associated with clinical pregnancy in the vitrified cleavage-stage embryo transfer cycles, and a single factor (the top-quality blastocyst score at transfer) was associated with clinical pregnancy in the vitrified blastocyst transfer cycles.
In cryopreserved cleavage-stage embryo transfer cycles, further cleavage or mitosis resumption during overnight culture has been reported to be associated with the clinical pregnancy rate [12-15]. In those studies, the embryos were usually assessed to determine whether further cleavage occurred. In the present study, the embryos were scored quantitatively at the time of warming and transfer. We found that the degree of post-warming embryo development and the top-quality embryo score at transfer were factors that significantly affected clinical pregnancy. Our finding suggests that embryos showing active cleavage events during overnight culture could have better implantation potential. Although an association between women’s age and clinical pregnancy has been reported previously, we did not find such a relationship [5,6].
In cryopreserved blastocyst transfer cycles, it has been reported that the inner cell mass or trophectoderm grade at the time of freezing or warming was associated with the clinical pregnancy rate [8-10]. However, in the present study, we assessed blastocysts using a single scoring system that considered three components simultaneously: inner cell mass grade, trophectoderm grade, and blastocyst developmental stage. This single scoring system is especially useful when two or more blastocysts are transferred. Furthermore, we assessed the blastocyst score at both warming and transfer. Although the majority of blastocysts develop during overnight culture, we found that the degree of post-warming blastocyst development was not associated with clinical pregnancy. Only the top-quality blastocyst score at transfer was associated with clinical pregnancy.
If embryos or blastocysts survive after warming, they can degenerate (i.e., the cell number decreases or they are downgraded) during overnight culture; thus, the embryo score could decline. In the present study, the degeneration rate was 3.2% per embryo and 6.7% per blastocyst. In a prior study, the embryo degeneration rate during overnight culture was 0.67% [7]. Our degeneration rate was rather high, but we obtained an acceptable pregnancy rate. As shown in our results, pregnancy was closely associated with the top-quality embryo or blastocyst score at the time of transfer.
A limitation of our study is that we analyzed relatively few cases of vitrified embryo or blastocyst transfer cycles. However, to the best of our knowledge, this is the first report to demonstrate that the degree of post-warming embryo development was associated with clinical pregnancy. A top-quality embryo score at transfer of ≥ 60.0 and a difference in the score between warming and transfer of ≥ 23.0 showed similar predictive performance for forecasting clinical pregnancy in vitrified cleavage-stage embryo transfer cycles. In blastocyst transfer cycles, the top-quality blastocyst score at transfer was the only significant predictor of clinical pregnancy.
Notes
Conflict of interest
Byung Chul Jee has been an editor of Journal of Clinical and Experimental Reproductive Medicine since 2018; however, he was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Figure 1.
A receiver operating characteristic (ROC) curve analysis demonstrating that the top-quality embryo score at transfer could predict clinical pregnancy in vitrified embryo transfer cycles (cutoff ≥ 60.0; area under the curve, 0.673; 95% confidence interval, 0.531– 0.815).
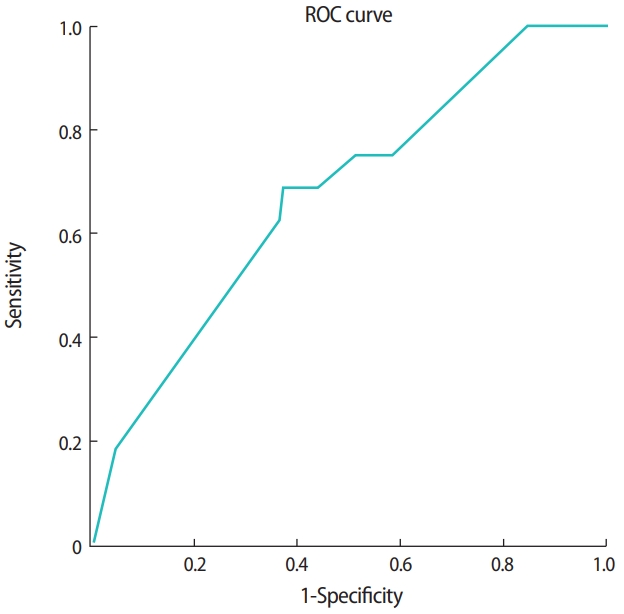
Figure 2.
A receiver operating characteristic (ROC) curve analysis demonstrating that the change (Δ) in the top-quality embryo score between warming and transfer could predict clinical pregnancy in vitrified embryo transfer cycles (cutoff ≥ 23.0; area under the curve, 0.675; 95% confidence interval, 0.514–0.835).

Figure 3.
A receiver operating characteristic (ROC) curve analysis demonstrating that the top-quality blastocyst score at transfer could predict clinical pregnancy in vitrified blastocyst transfer cycles (cutoff ≥ 38.3; area under the curve, 0.666; 95% confidence interval, 0.525– 0.807).
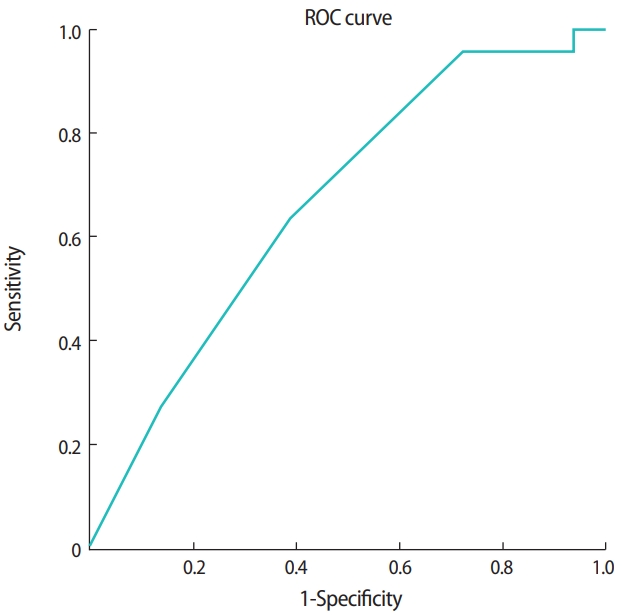
Table 1.
Basal characteristics and cycle outcomes in vitrified cleavage-stage embryo transfer cycles
Table 2.
Receiver operating characteristic curve analysis for the prediction of clinical pregnancy in vitrified cleavage-stage embryo transfer cycles
Table 3.
Basal characteristics and cycle outcomes in vitrified blastocyst transfer cycles
Table 4.
Receiver operating characteristic curve analysis for the prediction of clinical pregnancy in vitrified blastocyst transfer cycles
References
1. Gerris J, De Neubourg D, De Sutter P, Van Royen E, Mangelschots K, Vercruyssen M. Cryopreservation as a tool to reduce multiple birth. Reprod Biomed Online 2003;7:286-94.


2. Pandian Z, Templeton A, Serour G, Bhattacharya S. Number of embryos for transfer after IVF and ICSI: a Cochrane Review. Hum Reprod 2005;20:2681-7.



3. Wong KM, Mastenbroek S, Repping S. Cryopreservation of human embryos and its contribution to in vitro fertilization success rates. Fertil Steril 2014;102:19-26.


4. Ghobara T, Gelbaya TA, Ayeleke RO. Cycle regimens for frozenthawed embryo transfer. Cochrane Database Syst Rev 2017;7:CD003414.


5. Wang JX, Yap YY, Matthews CD. Frozen-thawed embryo transfer: influence of clinical factors on implantation rate and risk of multiple conception. Hum Reprod 2001;16:2316-9.



6. Salumets A, Suikkari AM, Makinen S, Karro H, Roos A, Tuuri T. Frozen embryo transfers: implications of clinical and embryological factors on the pregnancy outcome. Hum Reprod 2006;21:2368-74.



7. Goto S, Kadowaki T, Tanaka S, Hashimoto H, Kokeguchi S, Shiotani M. Prediction of pregnancy rate by blastocyst morphological score and age, based on 1,488 single frozen-thawed blastocyst transfer cycles. Fertil Steril 2011;95:948-52.


8. Honnma H, Baba T, Sasaki M, Hashiba Y, Ohno H, Fukunaga T, et al. Trophectoderm morphology significantly affects the rates of ongoing pregnancy and miscarriage in frozen-thawed singleblastocyst transfer cycle in vitro fertilization. Fertil Steril 2012;98:361-7.


9. Zhang H, Zhou Y, Li Y, Zheng Y, Xiao S, Wu Y, et al. Prediction of clinical pregnancy in vitrified-warmed single blastocyst transfer cycles by pre-freeze morphology. Iran J Reprod Med 2014;12:567-72.


10. Irani M, Reichman D, Robles A, Melnick A, Davis O, Zaninovic N, et al. Morphologic grading of euploid blastocysts influences implantation and ongoing pregnancy rates. Fertil Steril 2017;107:664-70.


11. Hur YS, Ryu EK, Song SH, Yoon SH, Lim KS, Lee WD, et al. A retrospective study of single frozen-thawed blastocyst transfer. Clin Exp Reprod Med 2016;43:106-11.




12. Van der Elst J, Van den Abbeel E, Vitrier S, Camus M, Devroey P, Van Steirteghem AC. Selective transfer of cryopreserved human embryos with further cleavage after thawing increases delivery and implantation rates. Hum Reprod 1997;12:1513-21.



13. Ziebe S, Bech B, Petersen K, Mikkelsen AL, Gabrielsen A, Andersen AN. Resumption of mitosis during post-thaw culture: a key parameter in selecting the right embryos for transfer. Hum Reprod 1998;13:178-81.


14. Van Landuyt L, Van de Velde H, De Vos A, Haentjens P, Blockeel C, Tournaye H, et al. Influence of cell loss after vitrification or slowfreezing on further in vitro development and implantation of human day 3 embryos. Hum Reprod 2013;28:2943-9.



15. Fernandez Gallardo E, Spiessens C, D’Hooghe T, Debrock S. Effect of embryo morphology and morphometrics on implantation of vitrified day 3 embryos after warming: a retrospective cohort study. Reprod Biol Endocrinol 2016;14:40.



16. Ahlstrom A, Westin C, Wikland M, Hardarson T. Prediction of live birth in frozen-thawed single blastocyst transfer cycles by prefreeze and post-thaw morphology. Hum Reprod 2013;28:1199-209.



17. Shin JJ, Jeong Y, Nho E, Jee BC. Clinical outcomes of frozen embryo transfer cycles after freeze-all policy to prevent ovarian hyperstimulation syndrome. Obstet Gynecol Sci 2018;61:497-504.










