|
 |
- Search
| Clin Exp Reprod Med > Volume 42(3); 2015 > Article |
Abstract
Objective
Several publications have established a relationship between sperm DNA damage and male factor infertility, based on data from America, Europe, and Asia. This study aimed to compare the extent of sperm DNA damage in sperm samples from Nigerian men with unexplained infertility and in sperm samples from a fertile group composed of sperm donors who had successfully impregnated a female partner naturally or through assisted conception.
Methods
A total of 404 men underwent male fertility evaluation at Androcare Laboratories and Cryobank participated in this study. Semen analysis and a sperm chromatin structure assay (SCSA) were performed on all subjects.
Results
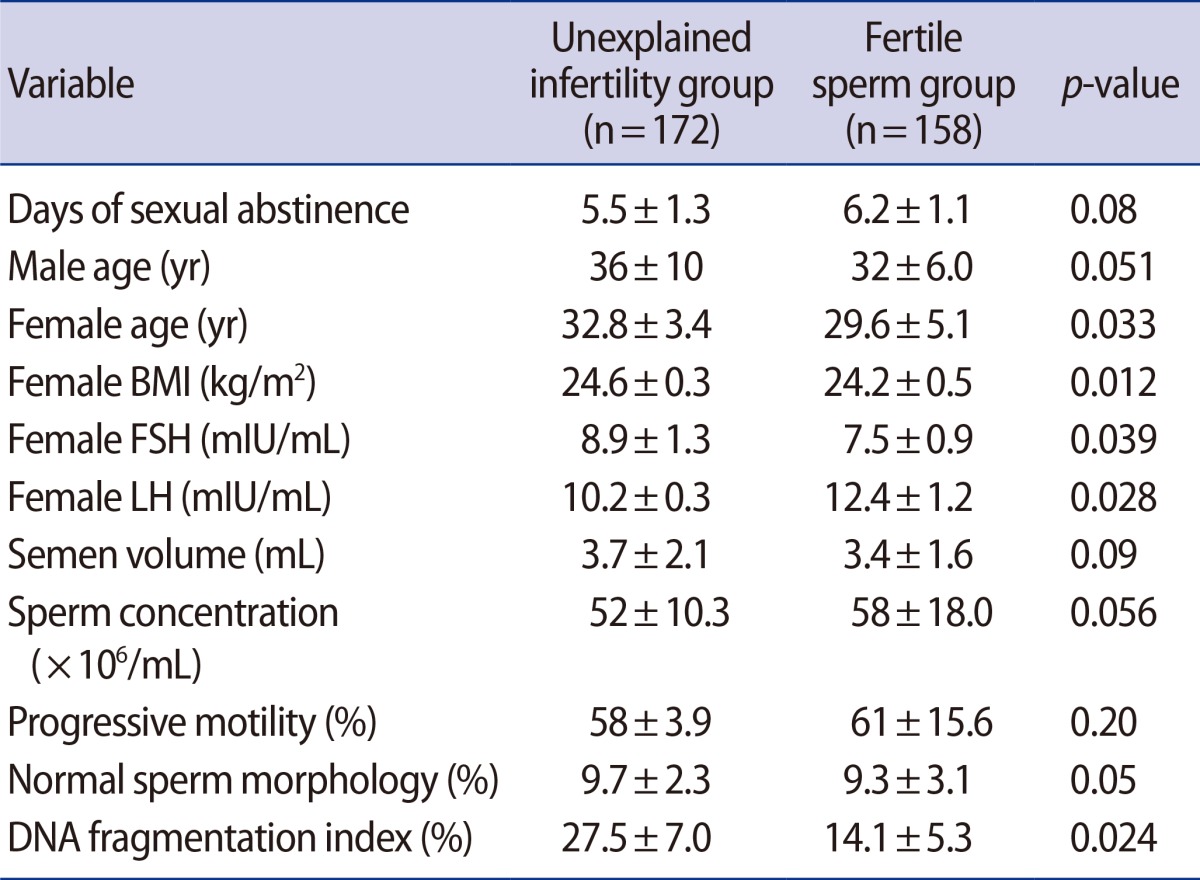
The men in the unexplained infertility group were slightly older than the men in the fertile sperm group (36┬▒10 years vs. 32┬▒6 years, p=0.051). No significant difference was observed between the two groups in semen analysis parameters (pŌēź0.05). Men in the unexplained infertility group with normal semen parameters had a significantly higher DNA fragmentation index (DFI) than men in the fertile sperm group (27.5%┬▒7.0% vs. 14.1%┬▒5.3%, p<0.05). In the unexplained infertility group, 63% of the men had a DFI greater than 20%, compared to 4% in the fertile sperm group. In the unexplained infertility group, 15.2% of the subjects had a DFI greater than 30%, compared to 1% in the fertile sperm group.
Infertility is defined as the inability to achieve pregnancy after 1 year of regular unprotected sexual intercourse [1]. Approximately 20% to 46% of married couples in sub-Saharan Africa, including Nigeria, are unable to achieve pregnancy successfully within 12 months of regular intercourse [2]. In 50% of the cases, the cause is attributed to the male partner [3]. A semen analysis is usually recommended to evaluate the fertility potential of the male partner. This test assesses parameters such as semen volume, sperm count (concentration), motility, and morphology [4]. Although this test has been helpful over the years in diagnosing male infertility, evidence suggests that it has failed to detect many causes of male factor infertility [5,6,7]. A pilot study of 28 infertile men with normal semen parameters found that 89.2% of the subjects had a high percentage of sperm DNA damage [8]. These findings indicate that men who have normal semen parameters can still be infertile. Most commonly, such men are classified as having unexplained infertility [9]. What is "unexplained" in this category of infertility is that tests have not been carried out to ascertain the quality of the sperm in terms of DNA integrity and ability to fertilize or interact with the cervical mucus and/or oocyte.
Sperm DNA integrity can be defined as the absence of fragmentation of the sperm DNA in the form of double or single strand breaks, which may occur at any stage from the transformation of the spermatogonia to the ejaculated spermatozoa [10]. The integrity of sperm DNA can be compromised by the following factors: apoptosis during spermatogenesis; strand breaks during chromatin remodeling and packaging; post-testicular DNA fragmentation induced by oxygen free radicals during transit through the male reproductive tract; DNA fragmentation induced by endogenous endonucleases; DNA damage induced by radiotherapy and chemotherapy; and DNA damage induced by environmental factors, such as smoking and air pollution [11]. Damage to sperm DNA integrity has been documented as a significant contributor to male factor infertility [12,13,14,15]. A number of tests have been developed to detect sperm DNA damage, including the terminal deoxynucleotidyl transferase-mediated dUDP nick end-labeling (TUNEL) assay, the single-cell gel electrophoresis (comet) assay, and the sperm chromatin structure assay (SCSA) [16,17,18].
The SCSA is the only one of these assays with clinically standardized cut-off values for predicting male fertility potential [19,20]. This procedure uses a flow cytometric test and SCSA software to evaluate approximately 5,000 to 10,000 sperm DNA breaks indirectly through DNA denaturability in few seconds [21].
In this study, the SCSA method was used to evaluate sperm DNA damage in Nigerian men with unexplained infertility. We examined the outcomes of the SCSA in semen samples categorized as normal according to the seminal analysis criteria of the World Health Organization (WHO), in couples who were unable to achieve pregnancy without assistance after 1 year of regular intercourse.
A total of 404 men 23 to 46 years of age who underwent male fertility evaluation at Androcare Laboratories and Cryobank from September 2013 to May 2015 participated in this study. Of the initial sample, 74 men were excluded from this study because the female partner failed to meet the inclusion criteria or the male partner had a history of chemotherapy, radiotherapy, chronic illness, or varicocele. Of the remaining participants, 172 were men with unexplained infertility, while 158 were men with normal semen parameters who sought to be sperm donors. This study was approved by the institutional review board of Androcare Laboratories and Cryobank. The sperm donors selected had a documented history of successfully impregnating a female partner naturally or through assisted conception. All participants were informed about this study and signed a consent form.
Participants were recommended to abstain from sexual intercourse for 2 to 5 days before the semen analysis. Semen was produced through masturbation in a 20-mL universal bottle within the laboratory premises. The analyses were performed within 30 minutes of ejaculation. A Makler chamber (Sefi-Medical Instruments, Haifa, Israel) was used to assess sperm count and motility. The semen parameters were classified according to the 2010 WHO criteria. Male partners with a semen analysis report below the WHO range for normospermia were excluded from subsequent evaluation.
The age of the female partner was documented, and each female partner underwent assessments including a hormonal assay, hysterosalpingography, and a baseline ultrasound. Hormonal assays were performed on the third day of the menstrual cycle, and only female partners with a follicle stimulating hormone level <10 mIU/mL, a luteinizing hormone level <15 mIU/L, and a body mass index <30 kg/m2 were included. A 15-mL plain tube was used to collect 10 mL of blood, which was analysed in a Stat Fax 4200 (Awareness Technology Inc., Palm City, FL, USA) after serum separation. Couples in which the female partner had a blocked fallopian tube, an abnormal hormonal profile or anovulation, a uterine disorder, endometriosis, cervical factor infertility, ovulatory factor infertility, peritoneal factor infertility, immunological factor infertility, or was above 37 years of age were excluded from this study.
Aliquots of the raw semen sample produced for analysis were separated in a 15-mL conical tube. The concentration of the sperm cells was diluted with Tris and NaEDTA buffer to 1-2├Ś106/mL, as previously described [15]. Then, 0.2 mL of the diluted semen was separated into a 5-mL round bottom tube and 0.4 mL of 0.08 M HCl (0.15 M NaCl, 0.1% Triton-X 100 pH 1.2, 4Ōäā) was immediately added. Thirty seconds later, 1.2 mL of acridine orange staining solution (0.037 M citric acid, 0.126 M Na2HPO4, 0.001 1 M disodium EDTA, 0.15 M NaCl pH 6.0, 4Ōäā) containing 6 g/mL of electrophoretically purified acridine orange was added and placed in the flow cytometer. The flow process was continued until 10,000 cells per sample were assessed. We used an epic XL flow cytometer (Beckman Coulter Inc., Miami, FL, USA) and SCSAsoft (SCSA Diagnostics, Brookings, SD, USA) to generate a native DNA/fragmented DNA cytogram, a total DNA stainability/DNA fragmentation index (DFI) scattergram, and a DFI frequency histogram. Based on these measurements, we were able to identify spermatozoa with high levels of DNA damage and stainability.
The DFIs of men with unexplained infertility and men in the fertile sperm group was determined. The percentage of men with a DFI at or above a given threshold in the two groups was compared using Fisher's exact test. Statistical analysis was performed using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). The p-values of less than 0.05 were considered to indicate statistical significance.
Men in the unexplained infertility group had a significantly higher DFI than men in the fertile sperm group (27.5%┬▒7.0% vs. 14.1%┬▒5.3%, p<0.05). The characteristics of the unexplained infertility group, the fertile sperm group, and their female partners are presented in Table 1. No significant differences were found between the two groups regarding seminal fluid parameters. In the unexplained infertility group, 63% of men had a DFI >20%, compared to the 4% in the fertile sperm group. In the unexplained infertility group, 15.2% of the men had a DFI greater than 30%, compared to 1% in the fertile sperm group.
In most cases of infertility, the workup is restricted to gynecological evaluation and semen analysis. Men are often categorized as fertile if their semen analysis is within the WHO normal range. A man classified as fertile by these poor predictive parameters may attribute the cause of their childlessness to the female partner. It is now becoming clear that high sperm DNA fragmentation may be the responsible factor in most couples with a history of unexplained infertility. Of the several mechanisms by which sperm DNA fragmentation can occur, the most devastating is post-testicular damage that occurs during sperm transport through the epididymis [11]. This damage is caused by high levels of reactive oxygen species produced by immature sperm that co-migrate with mature sperm.
Approximately 40% of all unexplained infertility cases have been reported to have a high DFI [21]. In our study, we found a DFI greater than 20% in 63% of men with unexplained infertility compared to 4% in the fertile sperm group. The DFI was greater than 30% in 15.2% of men with unexplained infertility compared to 1% in the fertile sperm group. DFI values between 20% and 30% have been associated with poor fertility potential [15,22]. Performing SCSA along with the traditional semen analysis could be helpful in predicting fertility potential. Performing SCSA could lead many men categorized as having unexplained infertility to make better decisions. For example, most couples with unexplained infertility are often advised to keep trying to achieve pregnancy naturally. This may go on for a number of years, and by the time they eventually decide to pursue assisted conception techniques such as intrauterine insemination (IUI) or in vitro fertilization, the chance of conceiving may be significantly affected by the female partner's age. In our study, we excluded female partners with a blocked fallopian tube, high body mass index, abnormal hormonal profile, uterine disorders, endometriosis, or who were above 37 years of age. This was done to avoid female factor infertility, which may have affected our results.
DFI values greater than 30% have been associated with poor or no chance of conceiving, even with IUI treatment [23]. In such men, SCSA could play a useful role, because they may be counselled to undergo direct intracytoplasmic sperm injection treatment [24], thereby saving valuable time and resources that would have been wasted trying IUI.
We did not find any significant difference in the semen analysis between the unexplained infertility group and the fertile sperm group. This highlights the poor predictive value of semen analysis compared to the SCSA. This is not surprising, since the WHO parameters only address a few aspects of sperm quality and function.
Our data indicated that sperm with high levels of DNA damage were more common in the unexplained infertility group than in the fertile sperm group (mean DFI values, 27.5%┬▒7.0% vs. 14.1%┬▒5.3%; p<0.05). The high levels of DNA damage in sperm in the unexplained infertility group may compromise embryo development in this group. This can be explained by the fact that development in early cleavage embryos is often driven by the maternal oocyte, and at this stage the male genome (sperm DNA) is silent [25]. Subsequent developments that require a concerted contribution from both the maternal and paternal genome may be affected adversely if the sperm DNA is damaged. The resulting embryo is more likely to experience developmental challenges, implantation failure, or miscarriage.
Our study showed that the SCSA is a more reliable predictor of fertility potential than traditional semen analysis in cases of unexplained infertility. Laboratory professionals and clinicians, especially in Nigeria and in Africa as a whole, may not have sufficient information regarding the strong evidence that links sperm DNA damage and infertility. Here, we present the SCSA as a useful predictor of fertility, especially in couples with a history of unexplained infertility.
Acknowledgments
We wish to acknowledge the efforts of Mrs. Mary Nkwoka, Mr. Fred Jibola and Dr. Paschal John for their technical assistance. We are also thankful to Dr. James Io and Mrs. Oluwakemi Faduola for their help in editing the manuscript.
References
1. Practice Committee of American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril 2013;99:63PMID: 23095139.


2. Idrisa A. Infertility. In: Kwawukume EY, Emuveyan EE, editors. Comprehensive gynaecology in the tropics. Accra: Graphic packaging; 2005. p. 333-343.
3. Thonneau P, Marchand S, Tallec A, Ferial ML, Ducot B, Lansac J, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989). Hum Reprod 1991;6:811-816.PMID: 1757519.


4. World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: WHO Press; 2010.
5. Aitken RJ, De Iuliis GN, McLachlan RI. Biological and clinical significance of DNA damage in the male germ line. Int J Androl 2009;32:46-56.PMID: 19076252.


6. Bonde JP, Ernst E, Jensen TK, Hjollund NH, Kolstad H, Henriksen TB, et al. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet 1998;352:1172-1177.PMID: 9777833.


7. van der Steeg JW, Steures P, Eijkemans MJ, F Habbema JD, Hompes PG, Kremer JA, et al. Role of semen analysis in subfertile couples. Fertil Steril 2011;95:1013-1019.PMID: 20338556.


8. Venkatesh S, Shamsi MB, Deka D, Saxena V, Kumar R, Dada R. Clinical implications of oxidative stress & sperm DNA damage in normozoospermic infertile men. Indian J Med Res 2011;134:396-398.PMID: 21985826.


9. Sigman M, Lipshultz LI, Howards SS. Office evaluation of the subfertile male. In: Lipshultz LI, Howards SS, Niederberger CS, editors. Infertility in the male. 4th ed. Cambridge: Cambridge University Press; 2009. p. 153-176.
10. Shamsi MB, Venkatesh S, Tanwar M, Talwar P, Sharma RK, Dhawan A, et al. DNA integrity and semen quality in men with low seminal antioxidant levels. Mutat Res 2009;665:29-36.PMID: 19427508.


11. Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril 2010;93:1027-1036.PMID: 20080235.


12. Henkel R, Hajimohammad M, Stalf T, Hoogendijk C, Mehnert C, Menkveld R, et al. Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertil Steril 2004;81:965-972.PMID: 15066449.


13. Evenson DP, Darzynkiewicz Z, Melamed MR. Relation of mammalian sperm chromatin heterogeneity to fertility. Science 1980;210:1131-1133.PMID: 7444440.


14. Tesarik J, Greco E, Mendoza C. Late, but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Hum Reprod 2004;19:611-615.PMID: 14998960.


15. Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, Purvis K, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod 1999;14:1039-1049.PMID: 10221239.


16. Morris ID, Ilott S, Dixon L, Brison DR. The spectrum of DNA damage in human sperm assessed by single cell gel electrophoresis (Comet assay) and its relationship to fertilization and embryo development. Hum Reprod 2002;17:990-998.PMID: 11925396.


17. Lewis SE, Agbaje IM. Using the alkaline comet assay in prognostic tests for male infertility and assisted reproductive technology outcomes. Mutagenesis 2008;23:163-170.PMID: 18325925.


18. Gorczyca W, Gong J, Darzynkiewicz Z. Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assays. Cancer Res 1993;53:1945-1951.PMID: 8467513.

19. Evenson DP, Larson KL, Jost LK. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl 2002;23:25-43.PMID: 11780920.


20. Erenpreiss J, Hlevicka S, Zalkalns J, Erenpreisa J. Effect of leukocytospermia on sperm DNA integrity: a negative effect in abnormal semen samples. J Androl 2002;23:717-723.PMID: 12185107.


21. Bungum M, Bungum L, Giwercman A. Sperm chromatin structure assay (SCSA): a tool in diagnosis and treatment of infertility. Asian J Androl 2011;13:69-75.PMID: 21057512.


22. Spano M, Bonde JP, Hjollund HI, Kolstad HA, Cordelli E, Leter G. Sperm chromatin damage impairs human fertility: the Danish First Pregnancy Planner Study Team. Fertil Steril 2000;73:43-50.PMID: 10632410.


23. Bungum M, Humaidan P, Spano M, Jepson K, Bungum L, Giwercman A. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum Reprod 2004;19:1401-1408.PMID: 15117894.


24. Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, Erenpreiss J, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod 2007;22:174-179.PMID: 16921163.


25. Wong CC, Loewke KE, Bossert NL, Behr B, De Jonge CJ, Baer TM, et al. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol 2010;28:1115-1121.PMID: 20890283.


-
METRICS

- Related articles in Clin Exp Reprod Med
-
Cardiovascular risk may be increased in women with unexplained infertility2017 March;44(1)