|
 |
- Search
| Clin Exp Reprod Med > Volume 41(1); 2014 > Article |
Abstract
Objective
Estrogen related receptor ╬▓ (Esrrb) is a member of the orphan nuclear receptors and may regulate the expression of pluripotency-related genes, such as Oct4 and Nanog. Therefore, in the present study, we have developed a method for delivering exogenous ESRRB recombinant protein into embryos by using cell-penetrating peptide (CPP) conjugation and have analyzed their effect on embryonic development.
Methods
Mouse oocytes and embryos were obtained from superovulated mice. The expression of Oct4 mRNA and the cell number of inner cell mass (ICM) in the in vitro-derived and in vivo-derived blastocysts were first analyzed by real time-reverse transcription-polymerase chain reaction and differential staining. Then 8-cell embryos were cultured in KSOM media with or without 2 ┬Ąg/mL CPP-ESRRB protein for 24 to 48 hours, followed by checking their integration into embryos during in vitro culture by Western blot and immunocytochemistry.
Results
Expression of Oct4 and the cell number of ICM were lower in the in vitro-derived blastocysts than in the in vivo-derived ones (p<0.05). In the blastocysts derived from the CPP-ESRRB-treated group, expression of Oct4 was greater than in the non-treated groups (p<0.05). Although no difference in embryonic development was observed between the treated and non-treated groups, the cell number of ICM was greater in the CPP-ESRRB-treated group.
Various techniques have been introduced to satisfy the demand for suitable mammalian oocyte and embryo culture systems. For example, a co-culture system with somatic cells such as cumulus cells could increase the fertilization and blastocyst formation rate [1,2,3]. The addition of growth factors or cytokines that are secreted by reproductive organs to the culture medium also could increase embryonic development and implantation rate [4,5]. In fact, insulin-like growth factor-I (IGF-I) and IGF-II increase formation of blastocysts and their cell number, especially increasing the cell number of inner cell mass (ICM) [6,7], while leukemia inhibitory factor (LIF) increases blastocyst formation and trophoblast outgrowth [8]. However, even the usefulness of upgrading culture systems and the precise mechanisms of those materials in oocytes or embryos remain unclear. Furthermore, their relatively low stability should be overcome for general use in long-term culture systems.
Estrogen-related receptor ╬▓ (Esrrb) is a member of the orphan nuclear receptors. It is known to be a regulator of the expression of pluripotency-related genes such as Oct4 and Nanog, and maintains the undifferentiation and pluripotency of stem cells [9]. Oct4 also maintains the formation of the ICM, and it regulates blastocyst differentiation and embryonic development during peri-implantation [10]. Additionally, Esrrb plays an essential role in adequate placental development. In fact, knockdown of Esrrb induces differentiation of embryonic stem (ES) cells [11] and Esrrb-null mutant mice die at 10.5 days post conception due to placenta abnormality [12].
In a previous study, recombinant protein conjugated with cell penetrating peptide (CPP) using 7X-arginine (R7) was translocated into human mesenchymal stem cells or ES cells efficiently with biological activity [13,14]. In the present study, we have analyzed the in vitro development of mouse embryos by delivery of CPP-ESRRB recombinant protein into a culture system and developed a novel embryo culture system using exogenous protein delivery.
Four- to 5-week-old female and 8- to 10-week-old male B6D2F1 mice (Samtako, Seoul, Korea) were kept under controlled light and temperature conditions with free access to water and food under 12 hours light and 12 hours dark. All animal experiments were approved by the Animal Care and Use Committee of CHA University. The female mice were superovulated via intraperitoneal injection of 5 IU pregnant mare serum gonadotropin (PMSG, Sigma-Aldrich, St. Louis, MO, USA), followed by injection with 5 IU hCG after 48 hours (Sigma-Aldrich). The female mice were then mated with the male mice and their vaginal plugs were checked at 12 hours after mating.
Two-cell embryos were collected from the oviduct at 46 hours after hCG injection and cultured in potassium simple optimization medium amino acid (KSOM, Millipore, Billerica, MA, USA) under mineral oil (Sage, Trumbull, CT, USA) in an incubator at 37Ōäā and 5% CO2 in air. Then, 8-cell embryos derived in vitro were cultured in KSOM with or without 2 ┬Ąg/mL of CPP-conjugated ESRRB protein for 48 hours. At 120 hours after hCG injection, in vivo-derived blastocysts were recovered from the uterus and used as a control group.
Germinal vesicle-stage (GV) oocytes were collected from the ovaries at 48 hours after PMSG injection. Metaphase II (MII)-stage oocytes were collected after in vitro maturation of GV oocytes in maturation medium (KSOM containing 0.4% bovine serum albumin (BSA, Sigma-Aldrich) for 12 hours.
In order to link Esrrb to 7R (a CPP domain) and a 6 histidine (His)-tag, mEsrrb cDNA was inserted into a pET20b(+) vector to construct an expression plasmid according to the protocol of Jo et al. [15]. The CPP-ESRRB protein expression vector was transformed into BL21 (DE3) pLysS-competent cells (Stratagene Inc., La Jolla, CA, USA). Transformed cells were cultured in lysogeny broth (LB) medium with ampicillin and grown at 37Ōäā on a shaking plate. The protein synthesis was induced with 1 mM isopropyl-1-thio-╬▓-D-galactopyranoside (IPTG, Sigma-Aldrich) and cell cultures were further grown at 20Ōäā for 8 hours. The cells were lysed for 30 minutes at room temperature in a lysis buffer with NP-10 (50 mM NaH2PO4, Sigma-Aldrich); 300 mM NaCl (Sigma-Aldrich); and 10 mM imidazole (Sigma-Aldrich), pH 8.0; 1 mL of lysozyme (Sigma-Aldrich) solution; and benzonase (3 units/mL; Qiagen Inc., Basel, Switzerland). The lysate was then separated into supernatant and lysate pellets were obtained by centrifugation at 15,000 rpm. The soluble supernatant was purified using Ni-NTA Superflow Columns (Qiagen Inc.). The purified CPP-ESRRB protein was mixed with 2├Ś Laemmli sample buffer and boiled for 5 minutes for 15% SDS polyacrylamide gel electrophoresis (SDS-PAGE). Gels were stained with Coomassie Brilliant Blue R250 for visualization of the protein. The purified CPP-ESRRB protein was also confirmed by western blotting using ESRRB antibody.
The number of ICM and trophectoderm (TE) cells in the blastocysts derived from embryo experiments were counted by differential staining using two chromatin-specific fluorochromes with different fluorescent spectra: propidium iodide (Sigma-Aldrich), which enters only cells with damaged membranes, and bisbenzimide (Hoechst 33342, Sigma-Aldrich), which passes through both damaged and intact membranes. At day 5, the zona pellucida (ZP) of the collected blastocysts was removed by brief exposure to acid Tyrode's solution (Sigma-Aldrich). The ZP-free embryos were exposed to a 1:5 dilution of whole rabbit anti-mouse serum (Sigma-Aldrich) for 1 hour and washed three times with DPBS (Hyclone) containing 0.1% BSA for 5 minutes each. Then, the embryos were placed into a 1:10 dilution of guinea pig complement (Sigma-Aldrich) for 1 hour. Propidium iodide and bisbenzimide were added to the complement solution to a final concentration of 10 ┬Ąg/mL and 10 ┬Ąg/mL, respectively. Then, they were briefly washed in DPBS (Hyclone) containing 0.1% BSA (Sigma-Aldrich) and were mounted between the slide and coverslip and examined under ultraviolet light using an Axio Imager A2 microscope (Carl Zeiss, Jena, Germany) fitted for epifluorescence. The nuclei of the ICM were labeled with bisbenzimide and appeared blue, while the nuclei of the TE cells appeared pink.
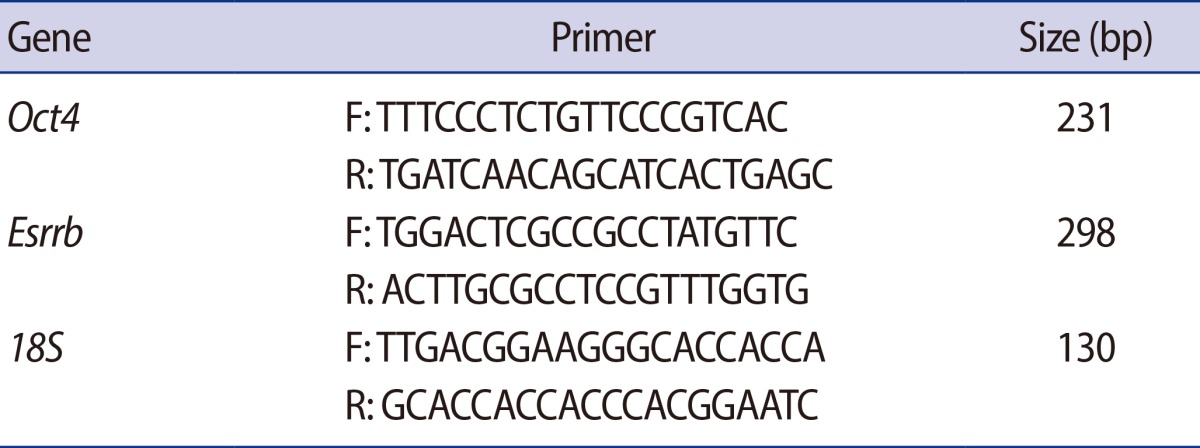
Total cellular RNA was extracted from 15 GV oocytes and embryos using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The first strand of cDNA was synthesized using a PrimeScript 1st strand cDNA Synthesis kit (Takara Bio, Shiga, Japan) according to the manufacturer's instructions. Reverse transcription polymerase chain reaction (PCR) was conducted using G-Tag (Cosmogene Tech, Seoul, Korea) and specific primer sets (Table 1). The cycle conditions were as follows: 94Ōäā for 5 minutes, followed by 35 cycles of a 94Ōäā denaturation period for 30 seconds, a 60Ōäā annealing period for 30 seconds, and a 72Ōäā elongation period for 40 seconds, with a final elongation period at 72Ōäā for 10 minutes. All PCR products were separated by 2% agarose gel electrophoresis. For quantification of the expression level, real-time PCR was performed by using iQTm SYBR Green super mix (Bio-Rad Laboratories, Hercules, CA, USA) on a Bio-Rad iQ5 real-time PCR machine. Each gene was normalized with 18S as housekeeping control. The results were analyzed by using the delta-delta Ct method with use of the 18S normalization control.
Western blot analysis was performed to characterize the CPP-ESRRB and confirm delivery of CPP-ESRRB protein into the embryos. Each sample containing 60 embryos, which were separated by 12% SDS-PAGE; after that, they were transferred onto polyvinylidene difluoride membranes (Bio-Rad Laboratories) stored for 90 minutes. Blocking was performed in DPBS containing 3% BSA and 0.1% Tween-20 (Sigma-Aldrich) for 2 hours at room temperature. The membranes were incubated in blocking solution with anti-ESRRB (Santa Cruz Biotech-nology, Santa Cruz, CA, USA, 1:200) or anti-His-tag (Sigma-Aldrich, 1:700) overnight, and then each sample was evaluated with a horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology) diluted to 1:2,000 with DPBS containing 0.1% Tween-20 (Sigma-Aldrich) for 1 hour. Immunoreactivity was detected using Western Blotting Luminol Reagent (Santa Cruz Biotechnology) and developed on Hyperfilm ECL X-ray film (Amersham Pharmacia Biotech, Buckinghamshire, UK).
Embryos cultured with or without CPP-ESRRB for 24 hours and 48 hours were fixed in 4% paraformaldehyde with 0.1% BSA (Sigma-Aldrich) at 4Ōäā for 30 minutes, and washed twice with DPBS (Hyclone) containing 0.1% BSA. After that, the embryos were permeabilized with 0.1% Triton X-100 (Sigma Aldrich) for 20 minutes and blocked in DPBS (Hyclone) with 3% BSA and 0.05% Tween-20 (Sigma-Aldrich) for 2 hours at 4Ōäā. They were then stained overnight at 4Ōäā with primary antibodies anti-His-taq (1:100, mouse polyclonal, Sigma-Aldrich) or anti-ESRRB (1:100, rabbit polyclonal, H70, Santa Cruz), followed by 2 hours incubation at 4Ōäā with Alexa Fluor 555-labeled goat anti-rabbit, 488-labeled goat anti-mouse secondary antibodies (Molecular Probes, Eugene, OR, USA) diluted to 1:200 with 3% BSA in DPBS (Hyclone). All of the samples were counterstained with 1 ┬Ąg/mL 4, 6-diamidino 2-phenyindiol (DAPI, Sigma Aldrich) diluted to 1:1000 with DPBS for 15 minutes at room temperature. All sample images were captured with an LSM 510 META confocal laser-scanning microscope (Carl Zeiss). The stained cell images were reconstructed using LSM 510 META software.
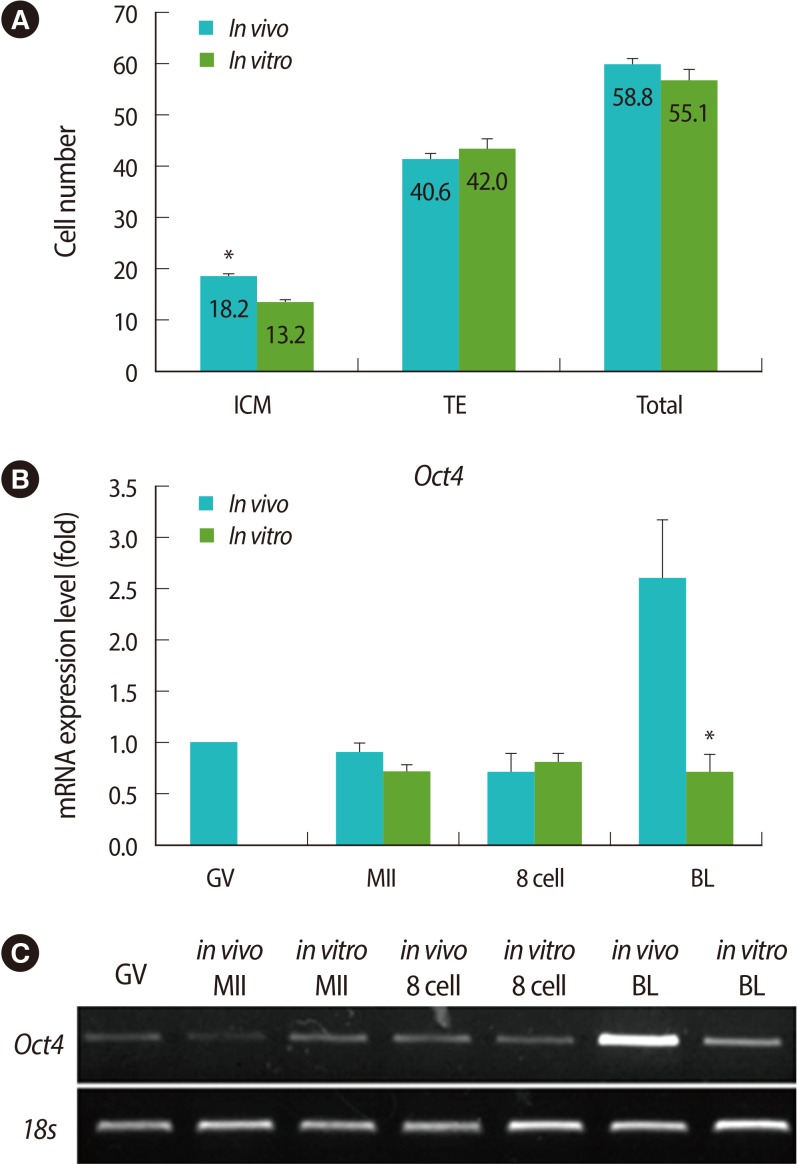
To verify the cell numbers of blastocysts derived from the groups cultured in vitro and in vivo, their cell numbers fo TE and ICM were counted using differential staining. The average cell number of ICM was lower in in vitro-derived blastocysts than in vivo-derived ones (Figure 1A). In addition, endogenous expression levels of Oct4, which regulates ICM formation, was also analyzed in the oocytes and embryos of each stage by real-time PCR. Like the cell number of ICM, the mRNA expression of Oct4 was lower in the in vitro-derived blastocysts than the in vivo-derived ones (Figure 1B, C).
The expression of CPP-ESRRB protein was induced by IPTG and was purified using His-tag at the C-terminal. The purified CPP-ESRRB protein was characterized through electrophoresis using SDS-PAGE gel and staining with Coomassie Brilliant blue R250 (Figure 3A). This result was reconfirmed by Western blot analysis using ESRRB-specific antibody (Figure 3B). A major single band was displayed, and that was CPP-ESRRB in 50 kDa (Figure 3B Lanes 2-4). A lower band was assumed to represent an additional protein, non-purified E. coli protein, or truncated CPP-ESRRB. In contrast, E. coli cell extracts containing the pET-empty construct did not display the major single band representing CPP-ESRRB-His (Figure 3A Lane 1, Figure 3B Lane 1). Figure 3 shows the high purity of the ESRRB isolation identified in this experiment.
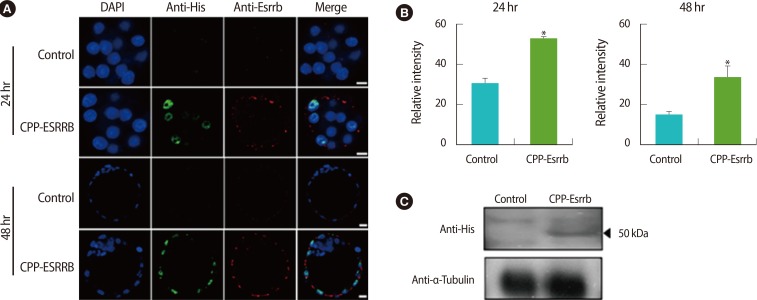
To observe the delivery of CPP-ESRRB recombinant protein into embryos during in vitro culture, we performed immunocytochemistry and western blot analysis. In immunocytochemical analysis using anti-His-tag and anti-ESRRB antibodies, the CPP-ESRRB delivered into the embryos was detected in their cytoplasm and nucleus at 24 hours and 48 hours after treatment, respectively (Figure 4A). However, their intensity was high at 24 hours after treatment and then slightly decreased at 48 hours (Figure 4B). Western blot analysis using anti-His-tag antibody also clearly showed that CPP-ESRRB recombinant protein was delivered in embryos of the treated group (Figure 4C).
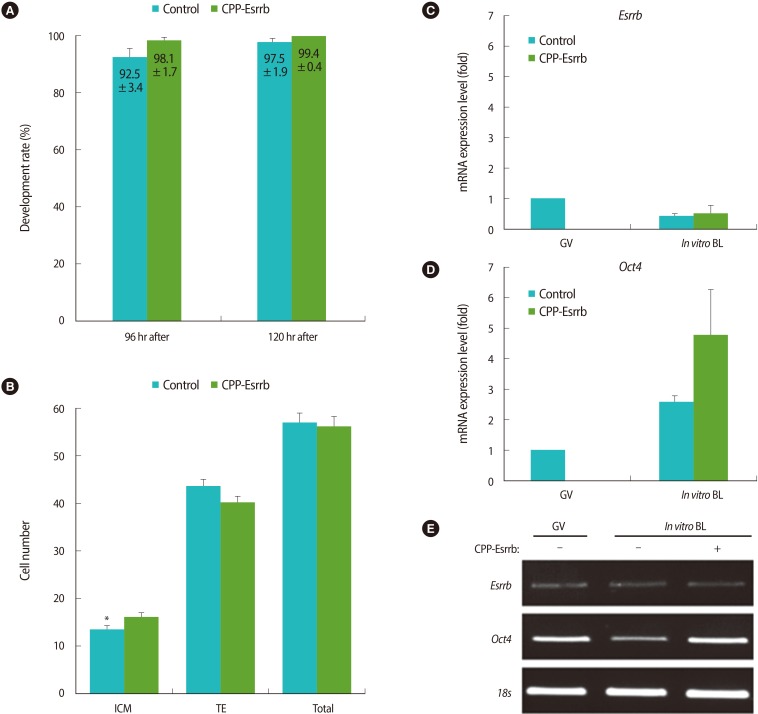
As shown in the Figure 5A, treatment of CPP-ESRRB during in vitro culture was either performed or not; there were no differences in the rates of blastocyst formation between the two groups (98.1┬▒1.7% vs. 92.5┬▒3.4% at 96 hours after hCG injection and 99.4┬▒0.4% vs. 97.5┬▒1.9% at 120 hours after hCG injection, respectively; p>0.05). However, the cell number of ICM in the CPP-ESRRB-treated embryos was greater than in the non-treated embryos (16.0┬▒1.1% vs. 13.4┬▒0.9%; p<0.05) (Figure 5B). Interestingly, there were no significant differences in the cell number of total and TE in blastocysts obtained from both groups.
After the embryos were cultured in KSOM with or without 2 ┬Ąg/mL of CPP-ESRRB for 48 hours, in vitro grown blastocysts were collected from both groups and the expression of Esrrb and Oct4 mRNAs were analyzed by real-time PCR. As shown in Figure 5C, the expression level of endogenous Esrrb did not differ between the treated group and non-treated groups. However, blastocysts in the CPP-ESRRB-treated group have shown a higher expression of Oct4 mRNA compared to those of the non-treated group (Figure 5D, E).
In vitro culture systems for embryonic development are indispensable for performing assisted reproductive technology (ART) such as IVF, ICSI, and cryopreservation. However, the manipulation of embryos or oocytes under suboptimal conditions could cause abnormal development of the fetus and placenta [16]. Also, in fact, the cell number of blastocysts derived in vitro was lower than from those in vivo [17]. In the present study, we have found that the cell number of ICM and Oct4 mRNA expression of CPP-ESRRB-treated embryos was greater than those of non-treated embryos. Although we cannot completely exclude the possibility that other factors in the culture medium have affected embryonic development, we have shown that the addition of CPP-ESRRB to culture medium improved the quality of embryos in vitro.
According to recent reports, there are three mRNA alternative splicing isoforms of human Esrrb. The short form is located in the nucleus and activates transcription. The short form of human ESRRB is the actual human ortholog of mouse Esrrb [18]. ESRRB protein also has cysteine-rich domains that can combine with DNA for the regulation of transcription [19], and Esrrb was expressed within the nuclei of epithelial, stromal, immune, and endothelial cells [20]. Moreover, Esrrb regulates the expression of Oct4, and Oct4 was known to regulate Nanog as well, so these genes were regulated by each other [21]. In fact, Oct4-deficient embryos can develop to the blastocyst stage, but their ICM are not pluripotent [22]. Instead, they are restricted to differentiation along the extra embryonic trophoblast lineage. Therefore, Oct4 and Nanog are both required in vivo and in vitro for establishment and maintenance of pluripotent ICM in mouse blastocysts [23].
In the present study, we used a CPP protein delivery system known as protein transduction domains or membrane transduction peptides. They have been thought to be a useful tool due to their ability to translocate across cellular membranes [24]. They consist of short sequences of amino acids, such as arginine or lysine residues, which confer a positive charge to the CPPs [25]. We have chosen oligoarginine (R7), which does not enter the cell by endocytosis. Also, it can not only pass through the cell membrane easily, but also move throughout the nucleus [26]. As shown in Figure 4, we have found that CPP-conjugated 50 kDa ESRRB can successfully penetrate the ZP and cytoplasm of the embryo and move into the nucleus. In addition, treatment with CPP-ESRRB has not shown a negative effect on embryonic development, but can increase ICM formation during mouse embryogenesis (Figure 5). This suggests that CPP-ESRRB could manipulate some embryonic processes in other mammalian species that show poor ICM development under suboptimal culture conditions or after harsh ART procedures.
In conclusion, recombinant CPP-ESRRB could be easily delivered into embryos by CPP and may increase the level of Oct4 mRNA and the rate of ICM formation. This technology could also be a useful tool for improving culture systems in vitro. In further research, we should observe the implantation rate, full-term development, and second generation to confirm the stability of the culture system using CPP-ESRRB.
Notes
References
1. Goldberg JM, Khalifa EA, Friedman CI, Kim MH. Improvement of in vitro fertilization and early embryo development in mice by coculture with human fallopian tube epithelium. Am J Obstet Gynecol 1991;165:1802-1805.PMID: 1750478.


2. Freeman MR, Bastias MC, Hill GA, Osteen KG. Coculture of mouse embryos with cells isolated from the human ovarian follicle, oviduct, and uterine endometrium. Fertil Steril 1993;59:138-142.PMID: 8419201.


3. Saito H, Hirayama T, Koike K, Saito T, Nohara M, Hiroi M. Cumulus mass maintains embryo quality. Fertil Steril 1994;62:555-558.PMID: 8062952.


4. Paria BC, Dey SK. Preimplantation embryo development in vitro: cooperative interactions among embryos and role of growth factors. Proc Natl Acad Sci USA 1990;87:4756-4760.PMID: 2352946.



5. Morita Y, Tsutsumi O, Taketani Y. In vitro treatment of embryos with epidermal growth factor improves viability and increases the implantation rate of blastocysts transferred to recipient mice. Am J Obstet Gynecol 1994;171:406-409.PMID: 8059819.


6. Harvey MB, Kaye PL. Insulin-like growth factor-1 stimulates growth of mouse preimplantation embryos in vitro. Mol Reprod Dev 1992;31:195-199.PMID: 1554504.


7. Rappolee DA, Sturm KS, Behrendtsen O, Schultz GA, Pedersen RA, Werb Z. Insulin-like growth factor II acts through an endogenous growth pathway regulated by imprinting in early mouse embryos. Genes Dev 1992;6:939-952.PMID: 1317321.


8. Lavranos TC, Rathjen PD, Seamark RF. Trophic effects of myeloid leukaemia inhibitory factor (LIF) on mouse embryos. J Reprod Fertil 1995;105:331-338.PMID: 8568779.


9. Zhang X, Zhang J, Wang T, Esteban MA, Pei D. Esrrb activates Oct4 transcription and sustains self-renewal and pluripotency in embryonic stem cells. J Biol Chem 2008;283:35825-35833.PMID: 18957414.


10. Ovitt CE, Scholer HR. The molecular biology of Oct-4 in the early mouse embryo. Mol Hum Reprod 1998;4:1021-1031.PMID: 9835353.


11. Ivanova N, Dobrin R, Lu R, Kotenko I, Levorse J, DeCoste C, et al. Dissecting self-renewal in stem cells with RNA interference. Nature 2006;442:533-538.PMID: 16767105.


12. Luo J, Sladek R, Bader JA, Matthyssen A, Rossant J, Giguere V. Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-beta. Nature 1997;388:778-782.PMID: 9285590.


13. Jo J, Song H, Park SG, Lee SH, Ko JJ, Park JH, et al. Regulation of differentiation potential of human mesenchymal stem cells by intracytoplasmic delivery of coactivator-associated arginine methyltransferase 1 protein using cell-penetrating peptide. Stem Cells 2012;30:1703-1713.PMID: 22696466.


14. Choi S, Jo J, Seol DW, Cha SK, Lee JE, Lee DR. Regulation of pluripotency-related genes and differentiation in mouse embryonic stem cells by direct delivery of cell-penetrating peptide-conjugated CARM1 recombinant protein. Dev Reprod 2013;17:9-16.



15. Jo J, Lee Y, Oh MH, Ko JJ, Cheon YP, Lee DR. Up-regulation of pluripotency-related Genes in human amniotic fluid-derived stem cells by ESRRB conjugated with Cell-Penetrating Peptide. Dev Reprod 2010;14:243-251.
16. Farin CE, Farmer WT, Farin PW. Pregnancy recognition and abnormal offspring syndrome in cattle. Reprod Fertil Dev 2010;22:75-87.PMID: 20003848.


17. Kiessling AA, Davis HW, Williams CS, Sauter RW, Harrison LW. Development and DNA polymerase activities in cultured preimplantation mouse embryos: comparison with embryos developed in vivo. J Exp Zool 1991;258:34-47.PMID: 1869864.


18. Zhou W, Liu Z, Wu J, Liu JH, Hyder SM, Antoniou E, et al. Identification and characterization of two novel splicing isoforms of human estrogen-related receptor beta. J Clin Endocrinol Metab 2006;91:569-579.PMID: 16332939.


19. Giguere V, Yang N, Segui P, Evans RM. Identification of a new class of steroid hormone receptors. Nature 1988;331:91-94.PMID: 3267207.


20. Bombail V, MacPherson S, Critchley HO, Saunders PT. Estrogen receptor related beta is expressed in human endometrium throughout the normal menstrual cycle. Hum Reprod 2008;23:2782-2790.PMID: 18775884.



21. van den Berg DL, Zhang W, Yates A, Engelen E, Takacs K, Bezstarosti K, et al. Estrogen-related receptor beta interacts with Oct4 to positively regulate Nanog gene expression. Mol Cell Biol 2008;28:5986-5995.PMID: 18662995.



22. Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 1998;95:379-391.PMID: 9814708.


23. Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 2000;24:372-376.PMID: 10742100.


24. Patel LN, Zaro JL, Shen WC. Cell penetrating peptides: intracellular pathways and pharmaceutical perspectives. Pharm Res 2007;24:1977-1992.PMID: 17443399.


25. Kueltzo LA, Middaugh CR. Structural characterization of bovine granulocyte colony stimulating factor: effect of temperature and pH. J Pharm Sci 2003;92:1793-1804.PMID: 12949998.


26. Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988;55:1189-1193.PMID: 2849510.


Figure┬Ā1
Comparison of cell numbers and expression of Oct4 mRNA between in vitro- and in vivo-derived embryos. (A) The numbers of inner cell mass (ICM), trophectoderm (TE), and total cells in blastocysts derived from in vivo and in vitro cultivation were counted by differential staining. (B) The expression level of Oct4 in oocytes and embryos derived from in vivo and in vitro cultivation were analyzed by real-time reverse transcription-polymerase chain reaction (RT-PCR). (C) Representative images of real time RT-PCR. Data are shown as mean (%)┬▒SE for more than three replications. GV, germinal vesicle; MII, metaphase II; BL, blastocyst. *Significantly different (p<0.05).
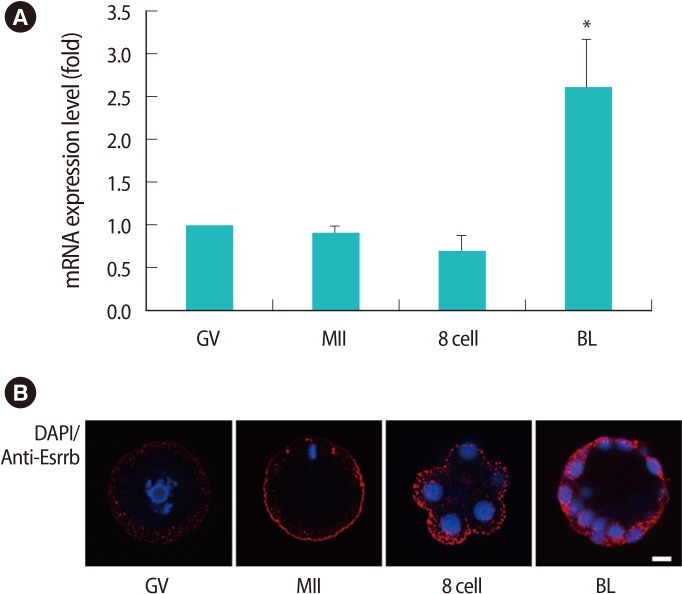
Figure┬Ā2
Expression patterns of estrogen related receptor ╬▓ (Esrrb) mRNA (A) and endogenous ESRRB in mouse oocytes and embryos (B). Data are shown as mean (%)┬▒SE for more than three replications. The scale bar is 5 ┬Ąm. DAPI, 4', 6-diamidino-2-phenylindole; GV, germinal vesicle; MII, metaphase II; BL, blastocyst. *Significantly different (p<0.05).
Figure┬Ā3
Characterization of recombinant cell-penetrating peptide-conjugated estrogen-related receptor ╬▓ (CPP-ESRRB). (A) Confirmation of pure isolation of CPP-ESRRB using staining with Coomassie Brilliant blue. (B) Confirmation of pure isolation of CPP-ESRRB by Western blot analysis using specific antibody against ESRRB.

Figure┬Ā4
Localization of recombinant cell-penetrating peptide-conjugated estrogen-related receptor ╬▓ (CPP-ESRRB) in mouse embryos according to treatment time. (A) Protein transduction of 7R-tagged ESRRB protein into embryos was detected by immunocytochemistry. CPP-ESRRB protein (2 ┬Ąg/mL) was added to the culture medium for the embryos. The embryos were fixed at 24 hours (morulas) and 48 hours (blastocysts) after treatment and then analyzed by immunostaining using 6├Ś His-taq and ESRRB antibodies. The nuclei were counterstained with 4', 6-diamidino-2-phenylindole (DAPI), and the images were merged. (B) The relative intensity was increased in the CPP-ESRRB-treated groups compared to the non-treated group. (C) Delivery of CPP-ESRRB protein into embryos was confirmed and analyzed by western blot analysis. The scale bars are 5 ┬Ąm. *Significantly different (p<0.05).
Figure┬Ā5
Comparison of cell numbers and expression of Oct4 mRNA between blastocysts treated with/without cell-penetrating peptide-conjugated estrogen-related receptor ╬▓ (CPP-ESRRB). (A) Blastulation rates in the CPP-ESRRB-treated group were compared with those from the non-treated one at different times after hCG injection. (B) The numbers of inner cell mass (ICM), trophectoderm (TE), and total cells in the blastocysts obtained from the CPP-ESRRB-treated group were compared with those from the non-treated one. (C, D) The expression levels of Esrrb and Oct4 in blastocysts obtained from the CPP-ESRRB-treated group were compared with those from the non-treated one. The data are shown as mean (%)┬▒SE for more than three replications. (E) Representative images of real-time reverse transcription-polymerase chain reaction. BL, blastocyst; GV, germinal vesicle. *Significantly different (p<0.05).