|
 |
- Search
| Clin Exp Reprod Med > Volume 40(4); 2013 > Article |
Abstract
Objective
To compare the mouse oocyte vitrification outcomes of the CryoLogic vitrification method (CVM) and the conventional open method using a Cryotop. Two CVM methods (original CVM and modified CVM) were tested.
Methods
Mature oocytes obtained from female BDF-1 mice were vitrified by two-step exposure to equilibrium and vitrification solutions. Three vitrification protocols were tested on three groups: the CVM-kit, modified CVM, and Cryotop groups. After exposure to the two solutions, the oocytes were vitrified. After warming, the oocytes were fertilized in vitro, and the embryo development was assessed. Blastomeres positive for caspase were counted using an in situ assay kit. The spindle morphology and chromosome configurations of warmed vitrified oocytes were also assessed.
Results
The modified CVM and Cryotop groups showed similar developmental capacities, and similar proportions of cells with intact spindles and chromosome configurations. The modified CVM protocol was superior to the original CVM protocol for developmental competence and intact spindle preservation. However, the CVM group showed a relatively higher number of apoptotic cells in blastocysts.
Oocyte cryopreservation is becoming an integral part of fertility preservation. Compared with slow freezing methods, vitrification is a simpler and more convenient strategy. It has conspicuously improved cryopreservation outcomes and pregnancy rates in humans after the transfer of frozen or vitrified oocytes [1-5].
Conventional vitrification methods usually place the specimen in direct contact with liquid nitrogen (LN2) during the vitrification process and for storage. Disease transmission through exposure to LN2 is therefore possible if oocytes, spermatozoa, or embryos contact contaminated LN2 [6,7]. The LN2 itself can be considered a potential source of pathogens during the vitrification procedure. Sterilization of LN2 in combination with an aseptic open vitrification system was reported to be a useful method for human oocyte vitrification [8,9]. However, there are potential risks when using an open vitrification system, even with sterile LN2. Thus, 'closed' systems have been developed to prevent germ cells or embryos from coming into direct LN2 contact.
Although 'closed' methods such as solid surface vitrification (SSV) or the open pulled straw method (OPS) potentially reduce pathogen contamination, lower survival rates have been reported in comparison to open methods such as with the Cryotop, which is the most widely used cryo-device [10].
A specific closed SSV system, the CryoLogic vitrification method (CVM), has been developed and employed for oocyte cryopreservation [11]. The CVM kit provides a nylon hook (CVM Fibreplug) and a metal block with a highly heat-conductive surface to vitrify an oocyte-containing microdrop. The CVM kit has been successfully used to vitrify porcine embryos [11], mouse oocytes [12], and mouse ovarian tissue and embryos [13,14]. The survival rate of the CVM-vitrified porcine embryos was relatively low compared with open vitrification methods such as the Cryotop. Although mouse oocytes showed a higher survival rate, it was still much lower than with open vitrification systems. In the present study, we aimed to compare the vitrification outcomes of mouse oocytes vitrified with closed CVM kit protocols (original CVM and modified CVM) to an open method using a Cryotop device. When we applied a modified CVM protocol during the vitrification process, the developmental competence of the preserved oocytes was improved relative to the original CVM protocol. Therefore, we compared three vitrification protocols in this study so as to develop an improved CVM protocol.
Five- to six-week-old female BDF-1 mice (Orient Co., Seoul, Korea) were used for the experiments. Animal care was conducted in accordance with the guidelines established by the Institutional Animal Care and Use Committee of Seoul National University Bundang Hospital.
Mice were treated intraperitoneally with injections of 5 IU pregnant mare's serum gonadotropin (Sigma Chemical, St. Louis, MO, USA) followed by 5 IU human chorionic gonadotropin (Sigma) intraperitoneally 48 hours later. The mice were sacrificed by cervical dislocation 13 to 14 hours after that, and the oviducts were excised and placed in 1 mL of washing medium (modified mouse tubal fluid, mMTF) supplemented with 0.4% (w/v) bovine serum albumin (BSA, Sigma). Cumulus oocyte complexes were released by tearing the ampulla of the oviducts. The cumulus cells were enzymatically removed using 85 IU/mL hyaluronidase (Cook, Brisbane, Australia) and mechanical dissociation with a glass pipette. Only morphologically normal mature Metaphase II oocytes, as judged by the presence of a first polar body, were used in our study.
The oocytes were suspended in an equilibrated solution (ES) containing 7.5% ethylene glycol (EG), 7.5% propanediol (PROH), and 20% fetal bovine serum (FBS, Sigma) in HEPES-buffered TCM-199 medium and incubated for 5 minutes. The oocytes were then transferred to vitrification solution (VS) containing 15% EG, 15% PROH, 0.5 M sucrose, and 20% FBS and incubated for 45 to 60 sonds at room temperature. In the CVM (CryoLogic, Victoria, Australia) groups, three to four oocytes were loaded onto the fine hook at the end of a plastic Fibreplug and immediately brought into contact with a metal surface or the wall of a hole in a metal block. Vitrified CVM carriers were plunged into LN2 and preserved until the warming process. In the Cryotop (Kitazato, Japan) group, four to five oocytes were loaded onto a Cryotop, which was then immediately plunged into LN2 for storage. For warming, the CVM Fibreplug or Cryotop was immersed directly in a 37Ōäā warming solution (1.0 M sucrose in 20% FBS-supplemented TCM-199) for 1 minute. The warmed oocytes were sequentially transferred to 0.5 M and 0.25 M sucrose in 20% FBS-supplemented TCM-199 for 3 minutes each, washed twice with washing medium (20% FBS in TCM-199), and transferred to culture medium at 37Ōäā in 5% CO2 in humidified air for 1 hour. Survival of the oocytes was assessed morphologically, based on plasma membrane integrity and discoloration of the ooplasm after the oocytes recovered from the warming procedure. The oocytes that survived were inseminated after the oocytes were cultured in IVF medium for 1 hour.
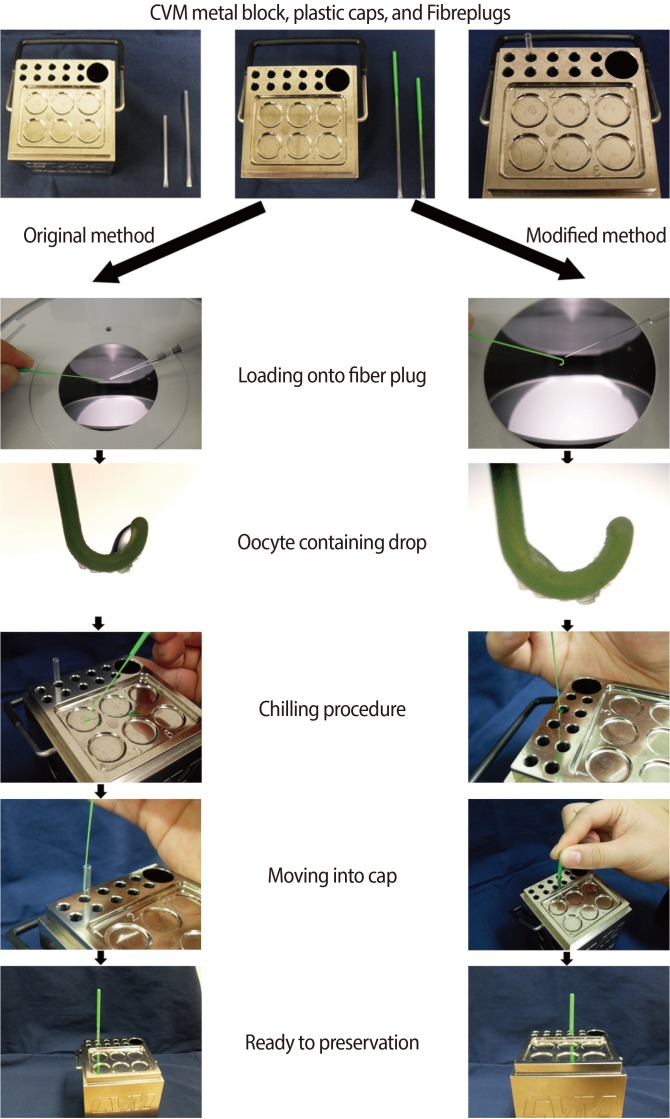
The manufacturer of the CVM kit offered the following protocol. After the oocytes were exposed to the vitrification media, a researcher held a pipette in one hand and a Fibreplug in the other. The aliquot at the end of the pipette tip was expelled and immediately transferred onto the hook of the Fibreplug. The Fibreplug was then carefully and quickly transferred to the CVM block and brought into contact with the vitrification surface located in a numbered well. Within a second, the droplet formed a glassy bead. The droplet was held against the vitrification surface for at least 10 seconds to chill the Fibreplug. The Fibreplug was then lifted off and inserted into a previously chilled sleeve. A researcher gently pushed the Fibreplug handle down into the sleeve. Finally, when vitrification was completed, the combined Fibreplug with sleeve was transferred from the small slot to a LN2-filled goblet, which was submerged in the cryocane. The goblet was capped and transported to the LN2 storage tank (Figure 1, left panel).
In the present study, we modified the manufacturer's suggested protocol. First, we used a thin glass pipette to minimize the VS volume (<0.2 ┬ĄL). Second, we transferred the Fibreplug to the metal wall of the hole instead of into the numbered well. The hole contained a previously chilled sleeve, and thus the time interval required for movement of the Fibreplug was shortened. The Fibreplug was held against the metal wall within the hole for at least 10 seconds before pushing the Fibreplug down into the sleeve. The remaining procedures were identical to the original protocol (Figure 1, right panel).
Epididymal spermatozoa were retrieved from the cauda epididymis of 8- to 10-week-old BDF-1 mice, and the sperm suspensions were pre-incubated for 1.5 hours in capacitation medium (mMTF supplemented with 0.8% BSA). The oocytes were then inseminated with sperm at a final dilution of 2├Ś106/mL and incubated at 37Ōäā in humidified 5% CO2 in air. After 6 hours, the inseminated oocytes were washed by pipetting and then placed into embryo maintenance medium (Global medium supplemented with 10% 50 mg/mL human serum albumin solution, LifeGlobal, Guilford, CT, USA). Fertilization was assessed by the formation of two-cell embryos on day 1 after insemination. The cleaved embryos were transferred to new embryo maintenance medium, and development to the blastocyst stage was assessed on day 5 after insemination.
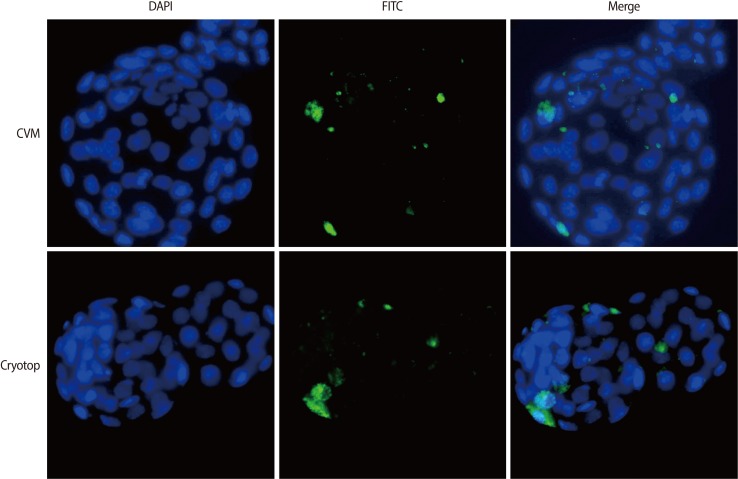
Some of blastocysts produced were stained using a commercial kit for caspase detection; we used a fluorochrome-labeled inhibitor of caspase (FLICA) in the CaspaTag Pan-Caspase in situ assay kit (Millipore, New Bedford, MA, USA). The FLICA staining solution was diluted (1:150) and centrifuged for 3 minutes at 3,000├Śg immediately before use. The washing buffer was diluted (1:10) in distilled water and stored at -20Ōäā for a maximum of 2 weeks. Positive controls were incubated in 0.1% H2O2 for 1 minute before staining. Immediately after washing in medium (0.1% polyvinyl alcohol [PVA] in PBS), positive controls were incubated in FLICA staining solution (35-┬ĄL droplets for 10 blastocysts) for 1 hour at 37Ōäā in 5% CO2 in air. Negative controls were incubated in PVA solution only. All of the blastocysts were washed in washing buffer for 5 minutes at room temperature and subsequently fixed in 300 ┬ĄL of the fixative included in the kit in a four-well dish for 15 minutes in the dark at room temperature. After fixation, the nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI) for 15 minutes. After a quick wash in 0.1% PVA containing PBS, the blastocysts were mounted on slides and observed using fluorescence microscopy. Blastomeres positive for caspase showed green fluorescence, and intact nuclei were stained blue. The ratio of green fluorescent blastomeres to the total number of nuclei was taken as the caspase-positive rate (Figure 2). For accuracy, three researchers counted the total blastomeres and caspase-positive blastomeres.
We performed spindle staining to evaluate the stability of each of the three vitrification tools. After vitrification and warming, some of the oocytes were incubated in fertilization medium (mMTF supplemented with 0.8% BSA) for 2 hours in 5.5% CO2 at high humidity at 37Ōäā and then fixed in 4% paraformaldehyde for 10 minutes. After fixation, the oocytes were washed in PBS and transferred to PBS containing 0.25% Triton X-100 for 10 minutes at room temperature. The oocytes were washed twice for 5 minutes each and blocked for 1 hour at room temperature in PBS containing 2% BSA. After rinsing in PBS, the oocytes were incubated overnight at 4Ōäā with a ╬▓-tubulin polyclonal antibody (Abcam, Cambridgeshire, UK) diluted 1:100 in PBS. After two PBS washes, the antibody-labeled microtubules were stained with fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG (Abcam) diluted (1:200) in PBS for 60 minutes in the dark at 37Ōäā. After washing in PBS, the samples were mounted onto a slide under a cover slip in Vectorshield mounting medium (Vector Laboratories, Burlingame, CA, USA), containing 0.5 ┬Ąg/mL DAPI. The localization patterns of tubulin and chromatin were revealed by FITC and DAPI fluorescence, respectively, as observed under 400├Ś magnification using a fluorescence microscope (Leica DMIL, Leica Microsystems GmbH, Ernst-leitz-Strasse, Germany) with a Hamamatsu digital imaging system. Typical barrel-shaped microtubules traversing the two poles and centrally aligned chromosomes were considered normal.
We expected problems with the original CVM kit protocol because temperature alteration occurs during the CVM vitrification procedure. Hence, we designed a modified CVM protocol. We simultaneously used and compared three vitrification methods (routine CVM, modified CVM, and Cryotop). After warming, each protocol was assessed for its rate of survival, fertilization, and blastocyst development. Apoptotic blastomeres were counted by the localization of blastocysts. We combined the total numbers of blastomeres and counted the apoptosis-positive cells using a merging program. In addition, we evaluated the spindle integrity for each group. For this experiment, we used another set of vitrified-warmed oocytes for staining of the spindles and chromosomes.
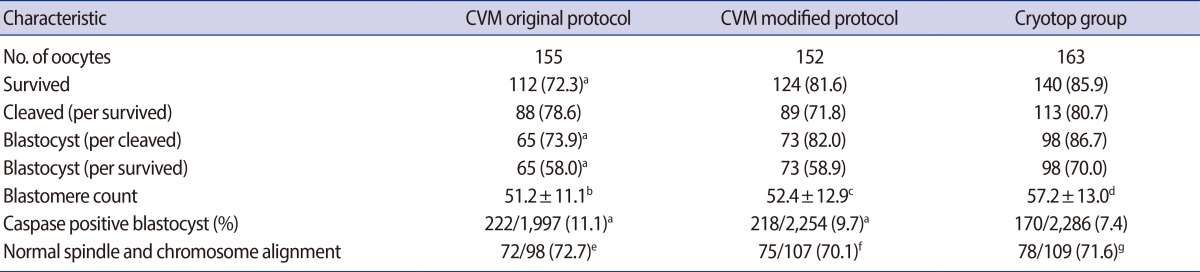
As shown in Table 1, the survival rate in the original CVM group was significantly lower than that in the Cryotop group. The cleavage rate after IVF was similar, but the blastocyst formation rate (per cleaved embryo) was significantly lower in the original CVM group than in the Cryotop group. The number of caspase-positive blastomeres was higher in the original CVM group than the Cryotop group.
We developed a modified CVM protocol in the following experiments. As presented in Table 1, the rates of post-warming survival, cleavage, and blastocyst formation (per cleaved embryo) were similar in the modified CVM protocol and the Cryotop group. However, the caspase-positive rate was higher in the modified CVM protocol.
Both the CVM and Cryotop groups showed similar rates of normal spindle morphology and chromosome alignment. Similar proportions of oocytes with normal spindle and chromosome morphology were observed in the original CVM, modified CVM, and Cryotop groups.
Cryopreservation of oocytes is a fertility preservation method in humans and in various animal species. An effective and advanced oocyte cryopreservation protocol would be a useful treatment option for women who are at risk of losing ovarian function because of pelvic diseases, surgery, or radio- and chemotherapy [15].
Promising results in clinical pregnancies and live births have been reported using conventional open vitrification protocols, such as the Cryotop, for human oocytes [16-21]. However, open methods may expose oocytes to pathogens that can survive in LN2. Although the risk of specimen contamination is relatively low, a potential hazard exists during storage periods. Recently, LN2 sterilization has been developed as a potentially useful method for avoiding cross-contamination. It was reported that LN2 sterilization could be performed by filtration or with UV irradiation [8,9,22]. Parmegiani et al. [23] investigated the safety of UV-LN2 sterilization and hermetical cryostorage of human oocytes. They demonstrated similar outcomes with respect to the rates of fertilization, cleavage, and top quality embryos between fresh and vitrified sibling oocytes of infertile patients. Although these methods may protect stored specimens from potential pathogens, it is clear that LN2 tanks can harbor microbes and may become contaminated during storage. Consequently, there is a need to ensure the absolute sterility of LN2 or to use barriers that prevent contact between LN2 and specimens.
In response to this need, Cobo et al. [3] demonstrated that vitrified human oocytes can be safely cryostored in LN2 vapor. They showed similar results between the vapor method and a direct LN2 contact protocol in the survival rate (95.3% vs. 94.5%), fertilization rate (73.1% vs. 71.7%), and blastocyst formation (54.7% vs. 53.9%) after human oocyte vitrification. However, even LN2 vapor protocols have the potential for cross-contamination.
To isolate oocytes from direct contact with LN2 during vitrification, various sealed-container systems have been developed [24-28]. Previous studies have demonstrated that closed methods such as closed pulled straws (CPS) [25], SSV [10], and CVM [12,13] have outcomes comparable to open methods. Previous reports also noted that closed methods were safe from microorganismal contamination of LN2. However, these methods have reduced cooling rates that may result in relatively lower rates of survival, fertilization, pregnancy, and live birth [12,13].
There have been many studies comparing open methods with closed protocols. Isachenko et al. [25] demonstrated that OPS and CPS methods have similar results for human oocyte vitrification, specifically, in the cleavage rate (62% vs. 65%) and blastocyst formation rate (15% vs. 14%) [25]. Sripunya et al. [10] compared the Cryotop to the SSV method and found similar results for survival (both groups over 90%), cleavage (78% vs. 64%), and blastocyst formation (12.3% vs. 10.3%). These findings showed that closed methods of oocyte vitrification are not inferior to open methods.
In the CVM protocol, oocytes are exposed to ES and VS and then transferred as a droplet onto the hook of the Fibreplug. The Fibreplug is then quickly transferred to a metal surface and inserted into a previously chilled sleeve (original protocol). During this procedure, some temperature changes within the vitrified droplets are possible during the transfer of the Fibreplug from the metal surface to a straw. According to Mazur and Seki [29], cooling and warming rates affect the survival rate of mouse oocytes. The rate of cooling was an essential factor in high survival. As described above, we were concerned about the negative effects of temperature changes in the standard CVM vitrification protocol and thus established a modified protocol.
In the present study, we used a modification of the original CVM protocol (Figure 1). First, we tried to minimize the solution volume (<0.2 ┬ĄL) during transfer of oocytes onto the Fibreplug by loading a relatively small number of oocytes and by using a thin glass pipette. We minimized the volume because larger volumes (the original protocol suggested over 1 ┬ĄL media volume per plug) might allow the formation of intracellular ice crystals. A small sample volume would also allow higher rates of cooling and warming. To achieve this small volume, we vitrified up to three oocytes at once. Second, we placed the Fibreplug in contact with the metal wall of the holes provided for the storage straws rather than in the wells on the surfaces of the metal block. As described above, the cooling rate could then be maintained during the vitrification procedure more effectively than in the manufacturer's suggested protocol. The exact cooling rates were not measured in this study; an altered cooling rate could have possibly been harmful to the oocytes when the original protocol was used. The original protocol suggested that the Fibreplug be moved into the sleeve after the plug was vitrified on a metal surface, which was a bit far away from the hole containing the sleeve. It was just a very short time during which the Fibreplug was covered with sleeve; at that moment, the slightly altered temperature might have been harmful to the oocytes' survival rate. In the vitrification procedure, a consistent temperature is very important to successful survival after warming. Hence, our modified CVM protocol should be beneficial to researchers.
In conclusion, we demonstrated that the closed vitrification method using a CVM device can be modified to achieve results similar to open vitrification using the Cryotop device with respect to survival, fertilization, blastocyst formation, and spindle integrity. Thus, closed vitrification using our modified CVM kit protocol may be used as an alternative to the conventional open method. However, further studies are needed to investigate the increased rate of caspase-positive blastomeres and the relatively lower rate of cleavage with the modified CVM kit protocol.
Notes
References
1. Ubaldi F, Anniballo R, Romano S, Baroni E, Albricci L, Colamaria S, et al. Cumulative ongoing pregnancy rate achieved with oocyte vitrification and cleavage stage transfer without embryo selection in a standard infertility program. Hum Reprod 2010;25:1199-1205.PMID: 20185513.



2. Cobo A, Meseguer M, Remohi J, Pellicer A. Use of cryo-banked oocytes in an ovum donation programme: a prospective, randomized, controlled, clinical trial. Hum Reprod 2010;25:2239-2246.PMID: 20591872.


3. Cobo A, Romero JL, Perez S, de los Santos MJ, Meseguer M, Remohi J. Storage of human oocytes in the vapor phase of nitrogen. Fertil Steril 2010;94:1903-1907.PMID: 20138272.


4. Nagy ZP, Chang CC, Shapiro DB, Bernal DP, Kort HI, Vajta G. The efficacy and safety of human oocyte vitrification. Semin Reprod Med 2009;27:450-455.PMID: 19806513.


5. Smith GD, Serafini PC, Fioravanti J, Yadid I, Coslovsky M, Hassun P, et al. Prospective randomized comparison of human oocyte cryopreservation with slow-rate freezing or vitrification. Fertil Steril 2010;94:2088-2095.PMID: 20171613.


6. Bielanski A, Bergeron H, Lau PC, Devenish J. Microbial contamination of embryos and semen during long term banking in liquid nitrogen. Cryobiology 2003;46:146-152.PMID: 12686204.


7. Bielanski A, Nadin-Davis S, Sapp T, Lutze-Wallace C. Viral contamination of embryos cryopreserved in liquid nitrogen. Cryobiology 2000;40:110-116.PMID: 10788310.


8. Parmegiani L, Cognigni GE, Filicori M. Ultra-violet sterilization of liquid nitrogen prior to vitrification. Hum Reprod 2009;24:2969PMID: 19749194.


9. Parmegiani L, Accorsi A, Cognigni GE, Bernardi S, Troilo E, Filicori M. Sterilization of liquid nitrogen with ultraviolet irradiation for safe vitrification of human oocytes or embryos. Fertil Steril 2010;94:1525-1528.PMID: 19591992.


10. Sripunya N, Somfai T, Inaba Y, Nagai T, Imai K, Parnpai R. A comparison of cryotop and solid surface vitrification methods for the cryopreservation of in vitro matured bovine oocytes. J Reprod Dev 2010;56:176-181.PMID: 19815985.


11. Beebe LF, Bouwman EG, McIlfatrick SM, Nottle MB. Piglets produced from in vivo blastocysts vitrified using the Cryologic Vitrification Method (solid surface vitrification) and a sealed storage container. Theriogenology 2011;75:1453-1458.PMID: 21220168.


12. Sanchez-Partida LG, Kelly RD, Sumer H, Lo CY, Aharon R, Holland MK, et al. The generation of live offspring from vitrified oocytes. PLoS One 2011;6:e21597PMID: 21738724.



13. Wang X, Catt S, Pangestu M, Temple-Smith P. Live offspring from vitrified blastocysts derived from fresh and cryopreserved ovarian tissue grafts of adult mice. Reproduction 2009;138:527-535.PMID: 19556437.


14. Wang X, Catt S, Pangestu M, Temple-Smith P. Successful in vitro culture of pre-antral follicles derived from vitrified murine ovarian tissue: oocyte maturation, fertilization, and live births. Reproduction 2011;141:183-191.PMID: 21075829.


15. Huang JY, Buckett WM, Gilbert L, Tan SL, Chian RC. Retrieval of immature oocytes followed by in vitro maturation and vitrification: a case report on a new strategy of fertility preservation in women with borderline ovarian malignancy. Gynecol Oncol 2007;105:542-544.PMID: 17379282.


16. Chian RC, Huang JY, Tan SL, Lucena E, Saa A, Rojas A, et al. Obstetric and perinatal outcome in 200 infants conceived from vitrified oocytes. Reprod Biomed Online 2008;16:608-610.PMID: 18492361.


17. Yoon TK, Kim TJ, Park SE, Hong SW, Ko JJ, Chung HM, et al. Live births after vitrification of oocytes in a stimulated in vitro fertilization-embryo transfer program. Fertil Steril 2003;79:1323-1326.PMID: 12798878.


18. Lucena E, Bernal DP, Lucena C, Rojas A, Moran A, Lucena A. Successful ongoing pregnancies after vitrification of oocytes. Fertil Steril 2006;85:108-111.PMID: 16412739.


19. Rienzi L, Romano S, Albricci L, Maggiulli R, Capalbo A, Baroni E, et al. Embryo development of fresh 'versus' vitrified metaphase II oocytes after ICSI: a prospective randomized sibling-oocyte study. Hum Reprod 2010;25:66-73.PMID: 19861328.


20. Katayama KP, Stehlik J, Kuwayama M, Kato O, Stehlik E. High survival rate of vitrified human oocytes results in clinical pregnancy. Fertil Steril 2003;80:223-224.PMID: 12849831.


21. Nagy ZP, Chang CC, Shapiro DB, Bernal DP, Elsner CW, Mitchell-Leef D, et al. Clinical evaluation of the efficiency of an oocyte donation program using egg cryo-banking. Fertil Steril 2009;92:520-526.PMID: 18692830.


22. Bielanski A, Vajta G. Risk of contamination of germplasm during cryopreservation and cryobanking in IVF units. Hum Reprod 2009;24:2457-2467.PMID: 19561041.


23. Parmegiani L, Cognigni GE, Bernardi S, Cuomo S, Ciampaglia W, Infante FE, et al. Efficiency of aseptic open vitrification and hermetical cryostorage of human oocytes. Reprod Biomed Online 2011;23:505-512.PMID: 21843968.


24. Kuleshova LL, Shaw JM. A strategy for rapid cooling of mouse embryos within a double straw to eliminate the risk of contamination during storage in liquid nitrogen. Hum Reprod 2000;15:2604-2609.PMID: 11098034.


25. Isachenko V, Montag M, Isachenko E, Zaeva V, Krivokharchenko I, Shafei R, et al. Aseptic technology of vitrification of human pronuclear oocytes using open-pulled straws. Hum Reprod 2005;20:492-496.PMID: 15528262.


26. Cremades N, Sousa M, Silva J, Viana P, Sousa S, Oliveira C, et al. Experimental vitrification of human compacted morulae and early blastocysts using fine diameter plastic micropipettes. Hum Reprod 2004;19:300-305.PMID: 14747171.


27. Kuwayama M, Vajta G, Ieda S, Kato O. Comparison of open and closed methods for vitrification of human embryos and the elimination of potential contamination. Reprod Biomed Online 2005;11:608-614.PMID: 16409712.


28. Vanderzwalmen P, Zech N, Prapas Y, Panagiotidis Y, Papatheodorou A, Lejeune B, et al. Closed carrier device: a reality to vitrify oocytes and embryos in aseptic conditions. Gynecol Obstet Fertil 2010;38:541-546.PMID: 20800527.


29. Mazur P, Seki S. Survival of mouse oocytes after being cooled in a vitrification solution to -196 degrees C at 95 degrees to 70,000 degrees C/min and warmed at 610 degrees to 118,000 degrees C/min: a new paradigm for cryopreservation by vitrification. Cryobiology 2011;62:1-7.PMID: 21055397.


Figure┬Ā1
Two vitrification procedures using the CVM kit: the original protocol (left panel) and the modified protocol (right panel). CVM, CryoLogic vitrification method.