 |
 |
- Search
| Clin Exp Reprod Med > Volume 50(2); 2023 > Article |
|
Abstract
Objective
Despite our understanding of Sertoli cell function and the state of spermatogenesis, the underlying mechanisms remain unclear. This study was conducted to compare the effects of orchiectomy and steroid treatment on fertility in testicular atrophy occurring after testicular torsion.
Methods
Thirty-three rats were divided into four groups. The atrophy, orchiectomy, and atrophy-steroid groups each contained nine rats, while the control group contained six. The left testes were rotated 720┬║, and atrophy was observed. In the atrophy-steroid rats, orchiectomy was performed after atrophy, and 1 mg/kg steroid was injected. Each male rat was housed with five female rats for 6 days. The fertility of the male rats was evaluated based on the pregnancy of the female rats. Left and right orchiectomies were performed to determine the tissue Johnsen score (JS) and the serum inhibin B (IB) level.
Results
JS values were significantly lower in the atrophy, orchiectomy, and atrophy-steroid groups than in the control group (p<0.05), while no significant difference was observed in JS between the atrophy and orchiectomy groups (p>0.05). Similarly, no significant differences in IB level or fertility percentage were found between the atrophy and orchiectomy rats (p>0.05).
Testicular torsion (TT) causes severe testicular injury through cell membrane lipid peroxidation, protein denaturation, and DNA impairment [1]. Even after the diagnosis and treatment of TT, problems with fertility and testicular atrophy persist. The severity of testicular atrophy is exacerbated by a delay in surgical intervention. Researchers have considered whether testicular damage after TT, which negatively impacts spermatogenesis and fertility, depends on testicular anomalies or autoimmune mediators. Along these lines, the decision of whether to excise an atrophic testis is controversial [2].
The degree of infertility in cases of TT and testicular atrophy can range from oligospermia to azoospermia. Torsion causes spermatogenesis and Sertoli cells to deteriorate in the testicular tissue, and these changes can be identified based on histopathological examination. Johnsen score (JS) conveys detailed information regarding germ and Sertoli cells [2]. Some studies have shown that serum inhibin B (IB) levels reflect Sertoli cell function and the state of spermatogenesis [2-4].
Steroids prevent the production of reactive oxygen species by blocking the phospholipase A2 enzyme and inhibiting leukocyte activation. In this manner, they preserve the integrity of the cell membrane, inhibit an increase in capillary permeability, and exert an anti-inflammatory effect [5].
A prior study from our unit demonstrated for the first time that melatonin is a potent antioxidant agent in preventing testicular ischemia-reperfusion injury, as indicated by increased IB levels and JS values [2]. Although this finding enhanced our knowledge of Sertoli cell function and the state of spermatogenesis, many questions remain unanswered.
The current study was designed to determine the effectiveness of orchiectomy and steroid treatment on fertility after TT and to identify the more effective approach.
Local ethics committee approval was received before this study. The experimental protocol was approved by the Ethical Committee of Necmettin Erbakan University Scientific Research Council (2012-077). Six pubertal (75-day-old) and 27 prepubertal (45-day-old) male Wistar rats were used in this study [6]. The rats were housed in cages with four animals per cage on sawdust bedding and provided with standard rodent chow and water ad libitum. A constant temperature of 21 ┬║C and humidity of 55% were maintained, with 12-hour periods of light-dark exposure. A 3-month acclimatization period was allowed.
The animals were divided into four groups. The control group contained six pubertal male rats, while each of the three experimental groups (atrophy, orchiectomy, and atrophy-steroid treatment) contained nine prepubertal male rats. All surgical procedures were performed under ketamine anesthesia (50 mg/kg intramuscularly) with sterile technique. Surgery was conducted through a left ilioinguinal incision.
In the experimental groups, the left testis was rotated 720┬░ clockwise and fixed within the hemiscrotum with 4ŌĆō0 polyglactin suture. The skin was closed with 4ŌĆō0 silk suture, and the torsion was left in place for 40 days [7]. Atrophy was observed via inspection. After the rats recovered from surgery and reached the pubertal period, each was housed with five fertile female rats for 6 days.
The rats in the control group were also kept with five fertile female rats for 6 days. Rats were sacrificed via high-dose anesthetic agents after the birth of the pups. Later, left and right orchiectomies were performed. The tissue JS and the serum IB level were evaluated after orchiectomy. The fertility of the male rats was evaluated by identifying births and determining the pregnancy rates among the female rats (duration of pregnancy, 21┬▒2 days).
In the atrophy group, the left testis was dissected from the scrotum, and the TT procedure was performed [7]. Atrophy was observed by inspection. After the rats recovered from surgery and reached the pubertal period, each was housed with five fertile female rats for 6 days.
In the orchiectomy group, the torsion procedure was applied exactly as in the atrophy rats. Then, atrophy was observed by inspection, and left orchiectomy was performed. After the rats recovered from surgery and reached the pubertal period, each was housed with five fertile female rats for 6 days.
In the atrophy-steroid group, the torsion procedure was applied exactly as in the prior experimental groups. Then, 1 mg/kg steroid (methylprednisolone) (Prednol L; Mustafa Nevzat) was injected intramuscularly after orchiectomy following atrophy. Injections of methylprednisolone were continued once daily for 7 days for the atrophy-steroid group. In all groups, left orchiectomy was performed 40 days after torsion.
In the experimental groups, the rats were sacrificed after these procedures. Histopathologic examination was performed, and the JS in the testicular tissue was evaluated. A biochemical examination was performed, and serum IB levels were measured. The fertility of all male rats was evaluated by identifying births and determining the pregnancy rates among the female rats.
After blood samples were taken, serum IB levels were measured with an IB enzyme-linked immunosorbent assay kit (MyBioSource Inc.; catalog no.: MBS-162795).
All testes were fixed in Bouin solution for 24 hours, postfixed in 70% alcohol, and embedded in paraffin. Then, 5-┬Ąm sections were cut and stained with hematoxylin and eosin. Using a light microscope, histologic evaluation was performed by a pathologist in a blinded, randomly-numbered fashion without knowledge of which testes were experimentally torsed.
Testicular injury and spermatogenesis were graded as described by Johnson et al. [8]. JS was determined by evaluating four areas on four separate slides for each animal. A score of 1 was used to indicate a lack of seminiferous epithelial cells along with tubular sclerosis. A score of 2 indicated no germ cells, only Sertoli cells. A score of 3 indicated spermatogonia only, while a score of 4 indicated no spermatids, few spermatocytes, and arrest of spermatogenesis at the primary spermatocyte stage. A score of 5 indicated no spermatids and many spermatocytes. A score of 6 indicated no late and few early spermatids, arrest of spermatogenesis at the spermatid stage, and disturbance of spermatid differentiation. A score of 7 indicated no late and many early spermatids, a score of 8 indicated few late spermatids, and a score of 9 indicated many late spermatids and disorganized tubular epithelium. Finally, a score of 10 indicated full spermatogenesis.
The experimental model was very well tolerated in all groups, and no animals died during the experimental period. The mean IB activity levels for all groups are shown in Table 1. IB activity was significantly lower in the atrophy-steroid group than in the control, orchiectomy, and atrophy groups, whereas pairwise comparisons showed it was higher in the atrophy group than in the atrophy-steroid group (p<0.05). However, no significant differences in IB values were observed between the atrophy and orchiectomy groups (p>0.05).
Testicular injury and lower histologic scores were observed in rats in the atrophy, orchiectomy, and atrophy-steroid groups, but not in the control rats (Figure 1). The JS values in the three experimental groups (Figures 2-4) were significantly lower than in the control group (p<0.05). Testicular atrophy had developed in all testes in the three experimental groups, but JS was significantly higher in the control group than in the other groups (p<0.05) (Table 2). The histopathologic evaluation is summarized in Table 2.
The fertility of all male rats was evaluated by identifying births and determining the pregnancy rates among the female rats. The fertility evaluation is summarized in Table 3. Fertility percentages were significantly lower in all three experimental groups than in the control group (p<0.05); however, no significant differences were present between the fertility percentages of the atrophy and orchiectomy groups (p>0.05).
TT necessitates an aggressive surgical approach. Early surgical intervention increases the possibility of testicular salvage, but only 32% of testes can be saved [9].
The clinical applications of steroids include the treatment of hormone deficiency, inflammation suppression, immunosuppression, and the suppression of excess hormone secretion [10]. In an experimental study, Etensel et al. [11] reported that the contralateral testicular weight and volume in rats treated with torsion and detorsion, torsion with saline and detorsion, and dexpanthenol did not differ significantly from the control measurements. They suggested that dexpanthenol prevents testicular atrophy after 60 days of TT.
In our previous study, we found that once daily use of antioxidant agents for 7 days prevented testicular atrophy and was effective in testicular salvage [12]. Although this finding furthered our knowledge of the effects of antioxidants, many questions regarding infertility remained. To further clarify the effects of steroids, we chose to analyze testicular tissue specimens, blood, and fertility after steroid treatment.
We identified significantly lower JS values in the atrophy, orchiectomy, and atrophy-steroid groups compared to the control rats (p<0.05), whereas we observed no significant difference in JS between the atrophy and orchiectomy groups (p>0.05) in the ipsilateral testes. We also noted no significant differences in JS values among all groups (p>0.05) in the contralateral testes. No significant differences were present in IB level between the atrophy and orchiectomy groups (p>0.05). Fertility percentages were significantly lower in all three experimental groups than in the control group (p<0.05), but no significant difference was observed in fertility percentage between the atrophy and orchiectomy groups (p>0.05).
In unilateral testicular atrophy, which can occur in the prepubertal period due to various causes, orchiectomy does not appear to benefit fertility, as indicated by IB, JS, and fertility percentage.
Figure┬Ā1.
Photomicrograph of the control group showing the morphology of normal testicular tissue (Johnsen score, 9.500┬▒0.091) (H&E, ├Ś100).

Figure┬Ā2.
Photomicrograph of the atrophy group showing higher magnification of the morphology of atrophic testicular tissue (Johnsen score, 2.000┬▒0.147) (H&E, ├Ś100).
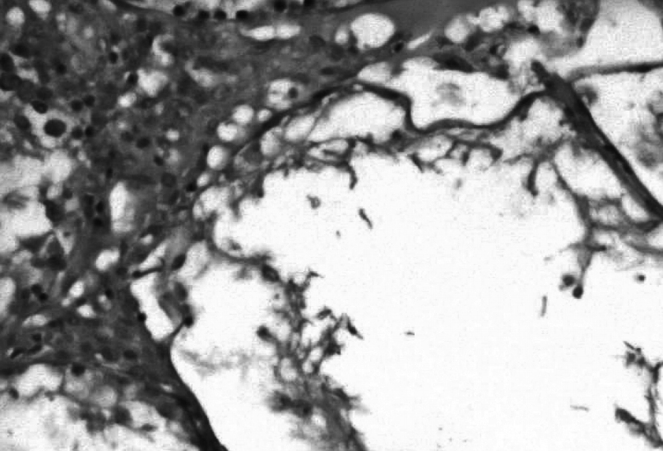
Figure┬Ā3.
Photomicrograph of the orchiectomy group showing higher magnification of the morphology of testicular tissue (Johnsen score, 1.556┬▒0.113) (H&E, ├Ś100).
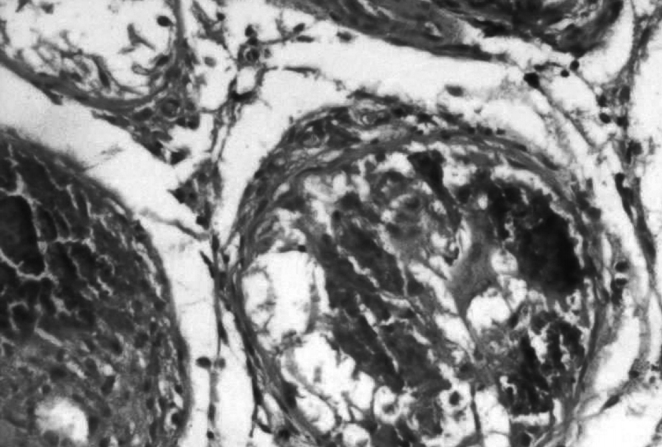
Figure┬Ā4.
Photomicrograph of the atrophy-steroid group showing higher magnification of the morphology of testicular tissue (Johnsen score, 1.000┬▒0.000) (H&E, ├Ś100).
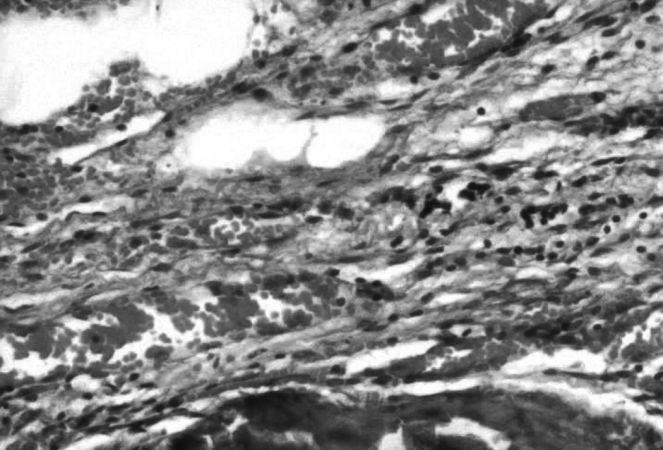
Table┬Ā1.
Serum inhibin B levels
| Group | Inhibin B level (pg/mL protein) |
|---|---|
| Control | 34.96┬▒0.53a),b) |
| Atrophy | 38.09┬▒0.46a) |
| Orchiectomy | 35.83┬▒0.39a) |
| Atrophy plus steroid | 31.79┬▒0.39b) |
References
1. Filho DW, Torres MA, Bordin AL, Crezcynski-Pasa TB, Boveris A. Spermatic cord torsion, reactive oxygen and nitrogen species and ischemia-reperfusion injury. Mol Aspects Med 2004;25:199-210.


2. Yurtcu M, Abasiyanik A, Avunduk MC, Muhtaroglu S. Effects of melatonin on spermatogenesis and testicular ischemia-reperfusion injury after unilateral testicular torsion-detorsion. J Pediatr Surg 2008;43:1873-8.


3. Kubini K, Zachmann M, Albers N, Hiort O, Bettendorf M, Wolfle J, et al. Basal inhibin B and the testosterone response to human chorionic gonadotropin correlate in prepubertal boys. J Clin Endocrinol Metab 2000;85:134-8.


4. Balci T, Kocabas R, Cuce G, Akoz M. Inhibition of fatty acid binding protein 4 in obese male mice adversely affects reproductive parameters. J Reprod Infertil 2021;22:16-22.




5. Jansen NJ, van Oeveren W, van den Broek L, Oudemans-van Straaten HM, Stoutenbeek CP, Joen MC, et al. Inhibition by dexamethasone of the reperfusion phenomena in cardiopulmonary bypass. J Thorac Cardiovasc Surg 1991;102:515-25.


6. Havenaar R, Meijer JC, Morton DB, Ritskes-Hoitinga J, Zwart P. Biology and husbandry of laboratory animals. In: van Zutphen LFM, Baumans V, Beynen AC, editors. Principles of laboratory animal science. Revised ed. Elsevier; 2001. p. 19-77.
7. Ayd─▒n A, Sonmez MG, Ecer G, Kilinc F, Kocabas R, Atilgan AE, et al. The effect of intratesticular dexpanthenol on experimentally-induced testicular ischaemia/reperfusion injury. J Pediatr Urol 2021;17:440.e1-7.


8. Johnson L, Petty CS, Neaves WB. The relationship of biopsy evaluations and testicular measurements to over-all daily sperm production in human testes. Fertil Steril 1980;34:36-40.


10. Brown DC. Adrenal corticosteroids, antagonists, corticotropin. In: Bennett PN, Brown MJ, Sharma Pankaj S, editors. Clinical pharmacology. 11th ed. Elsevier; 2012. p. 558-71.
- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 1,281 View
- 120 Download
- Related articles in Clin Exp Reprod Med
-
Effects of a short abstinence period on sperm quality in oligozoospermic men2023 December;50(4)
Effects of Calcium and dbcAMP on Sperm Motility in Mouse1989 ;16(2)






