Vitrification of mouse embryos using the thin plastic strip method
Article information
Abstract
Objective
The aim of this study was to compare vitrification optimization of mouse embryos using electron microscopy (EM) grid, cryotop, and thin plastic strip (TPS) containers by evaluating developmental competence and apoptosis rates.
Methods
Mouse embryos were obtained from superovulated mice. Mouse cleavage-stage, expanded, hatching-stage, and hatched-stage embryos were cryopreserved in EM grid, cryotop, and TPS containers by vitrification in 15% ethylene glycol, 15% dimethylsulfoxide, 10 µg/mL Ficoll, and 0.65 M sucrose, and 20% serum substitute supplement (SSS) with basal medium, respectively. For the three groups in which the embryos were thawed in the EM grid, cryotop, and TPS containers, the thawing solution consisted of 0.25 M sucrose, 0.125 M sucrose, and 20% SSS with basal medium, respectively. Rates of survival, re-expansion, reaching the hatched stage, and apoptosis after thawing were compared among the three groups.
Results
Developmental competence after thawing of vitrified expanded and hatching-stage blastocysts using cryotop and TPS methods were significantly higher than survival using the EM grid (p<0.05). Also, apoptosis positive nuclei rates after thawing of vitrified expanded blastocysts using cryotop and TPS were significantly lower than when using the EM grid (p<0.05).
Conclusion
The TPS vitrification method has the advantages of achieving a high developmental ability and effective preservation.
Introduction
In 1985, the first report of the successful birth of a mouse following cryopreservation of embryos by vitrification was published [1]. In the past decade, various new methods for embryo cryopreservation have been published [2]. Many investigators have also reported on the relative efficiency of cryopreservation according to the embryo culture environment, cryoprotectant modification, and cryopreservation container. Recent vitrification containers, such as electron microscope (EM) grids [3-6], open pulled straws (OPS) [7-12], open hemi-straws [13,14], cryoloops [15-19], and cryotops [20-23] have improved the development of vitrification, which has led to the successful production of offspring from vitrified-warmed embryos. However, the disadvantages of these containers include their sensitivity to time, proper training needed for technique, and cost [24-26].
The EM grid was the first container in which both a small sample volume and direct contact with liquid nitrogen (LN2) was achievable to obtain the very high cooling rates required for vitrification [3]. However, even these protocols with the EM grid have produced the formation of a vapor coat around the microdrop, as it directly drops onto the LN2. On the other hand, the cryotop has been developed for the vitrification of mammalian embryos [20]. The embryos with cryoprotectant can be loaded onto the strip. The sample is then immersed into LN2 for vitrification. This also allows for higher cooling, is easy to learn, and is simple to manipulate [27]. However, a disadvantage of this protocol with the cryotop is a greater expense compared to other vitrification containers.
Hence, we propose the use of the thin plastic strip (TPS) as a container for mouse embryo vitrification. The TPS vitrification container, consisting of polychlorotrifluoroethylene, is used to load a film of cryoprotectant containing the mouse embryos and enables facile manipulation during vitrification and warming. This method was modified from a procedure routinely used successfully with several other containers. The embryo is then directly immersed in LN2.
Confirming the effectiveness of vitrification containers, cryopreservation of mouse embryos has revealed developmental competence. In particular, apoptosis is crucially involved in development and differentiation. Environmental stress such as cryopreservation can induce unscheduled apoptosis during culture, which might lead to arrest or abnormal development and lower viability of embryos [28-30]. Therefore, embryo qualities are related to the ratio of the number of intact to damaged blastomeres, known as the apoptotic index. Poor grade embryos show a higher apoptotic index. The evaluation of DNA fragmentation by terminal deoxynucleotidyl transferase mediated dUTP nick end labeling (TUNEL) has been used as a reliable method for detection of apoptosis in embryos [31].
The objective of the present study was to evaluate the novel TPS vitrification method for the cryopreservation of mouse embryos followed by IVF. Also, the effect of TPS on embryonic development and the apoptosis rate in mouse embryos was examined.
Methods
1. Chemicals
Unless noted otherwise, all chemicals used in the present study were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
2. Ethical approval for scientific research
All animal care and use procedures were approved by the Institutional Animal Care and use Committee of Kangwon National University.
3. Embryo collection and culture
Six-week-old female imprinting control region mice were superovulated with an injection of 7.5 IU of pregnant mare serum gonadotropin (Invervet, Boxmeer, Netherlands) followed by 7.5 IU of hCG (Invervet) after 48 hours. After administration of the hCG, females were mated with males, and the copulation plugs were checked the following morning. Twenty hours after hCG injection, the female mice were sacrificed, and zygotes were collected with flushing from the oviduct. The one-cell embryos were denuded with hyaluronidase and washed two times in mouse tubal fluid (MTF) containing 4 mg/mL human serum albumin (Irvine Scientific, Santa Ana, CA, USA) and cultured into MTF medium at 37℃ in an atmosphere of 5% CO2. Sixty-four hours and 112 hours after hCG injection, vitrified cleavage and blastocyst stages, respectively, resulted.
4. Vitrification of embryos
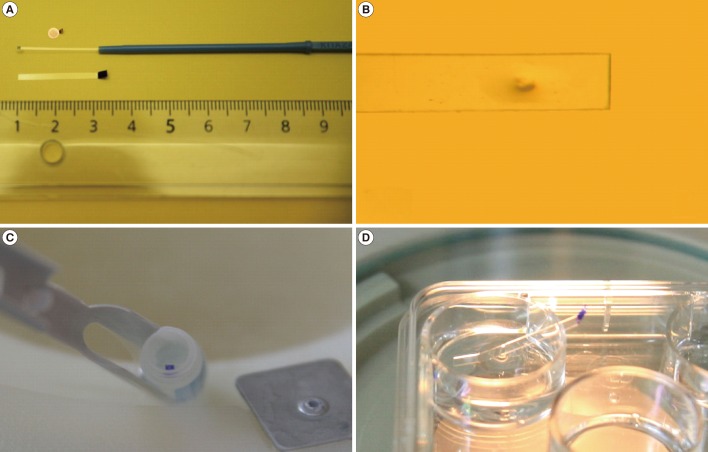
Vitrification of cleavage-stage embryos and blastocysts was performed as described previously [31,32]. Briefly, embryos were transferred into vitrification solution 1 (VS1) consisting of 7.5%% ethylene glycol (EG) and 7.5% dimethyl sulphoxide (DMSO) dissolved in Dulbecco's phosphate buffered saline (D-PBS) supplemented with 20% serum substitute supplement (SSS, Irvine Scientific) for 2 minutes in cleavage-stage embryos and 3 minutes in expanded, hatching, and hatched embryos. After an initial shrinkage, embryos were transferred into vitrification solution 2 (VS2) consisting of 15% EG, 15% DMSO, 10 µg/mL Ficoll, and 0.65 M sucrose dissolved in D-PBS supplemented with 20% SSS for 45 second. All the steps were performed at 37℃. After the exposure to VS2, embryos were quickly loaded into an EM grid, cryotop (Kitazato Ltd., Tokoyo, Japan), or our newly developed TPS container, and plunged into LN2. In the case of the expanded embryos, artificial shrinkage was performed using two 29-gauge needles. The TPS was manufactured by cutting a section 1.5 mm wide×25 mm long from polychlorotrifluoroethylene film (sheet thickness, 0.1 mm; SPL Life Science, Seoul, Korea) and equipped with a 5 minutes movable grip at the end. The loaded TPS container was then plunged into the LN2 using forceps and was subsequently transferred into a cryovial under LN2 (Figure 1).
5. Warming of embryos
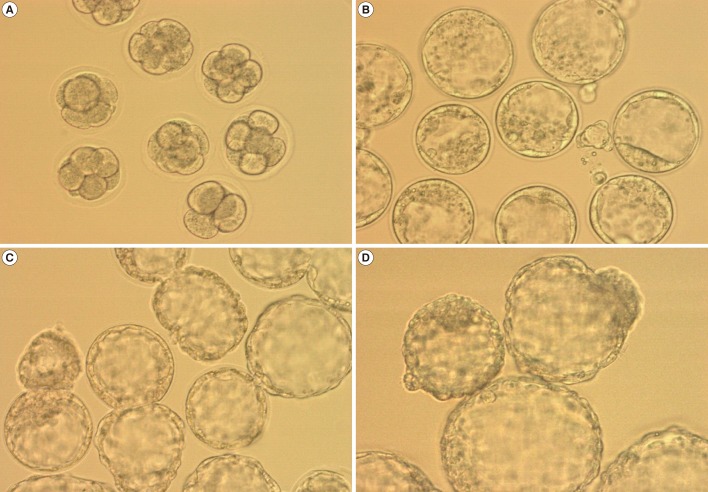
Each EM grid, cryotop, or TPS containing embryos was transferred for 2 minutes to warming solution 1 containing 0.25 M sucrose dissolved in D-PBS and supplemented with 20% SSS. The embryos were then transferred to warming solution 2 containing 0.125 M sucrose dissolved in DPBS supplemented with 20% SSS for 3 minutes. All of the steps were performed at 37℃. The embryos were then washed three times in MRC#46 medium (Biosupply, Seoul, Korea) and cultured in a multi-well dish for further culture at 37℃ in an atmosphere of 5% CO2. The post-thawing survival of the embryos was observed under an inverted microscope 3 to 72 hours after warming (Figure 2).
6. TUNEL assay
Embryos were fixed in 4% paraformaldehyde in PBS for 30 minutes at room temperature, followed by three washes in PBS containing 0.3% polyvinylalcohol (PVA). The embryos were permeabilized with 0.1% Triton X-100 for 10 minutes at 4℃, followed by three washes in PBS containing 0.3% PVA. The embryos were incubated in TUNEL reaction solution (Roche Applied Science, Mannheim, Germany) at 37℃ for 1 hour in the dark. The embryos were washed three times and mounted in 4', 6-diamidino-2-phenylindole (DAPI, Vector Laboratories, Burlingame, CA, USA). The embryos in the mounting drops were covered with a cover slip. Embryos were examined under a fluorescence microscope (Olympus, Tokyo, Japan) using the TUNEL assay and DAPI. The number of apoptotic nuclei and total number of nuclei were determined.
7. Statistical analyses
The different groups were compared using analysis of variance (Tukey's test) and chi-square test as needed for each case.
Results
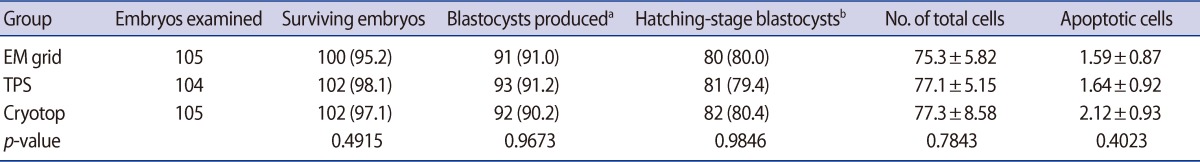
In the first series of experiments, we evaluated the effect of different container types (EM grid, TPS, and cryotop) during the vitrification of cleavage-stage mouse embryos. The survival, developmental competence, and apoptosis rates for cleavage-stage mouse embryos cryopreserved in the EM grid (n=105), cryotop (n=105), and TPS (n=104) containers are shown in Table 1. There were no significant differences among the three groups with regard to survival and developmental rates. The total number of cells and rate of cell apoptosis of cleavage-stage embryos vitrified-warmed on TPS (77.1±5.1 and 1.6±0.9) were also similar to those vitrified-warmed on an EM grid (75.3±5.8 and 1.5±0.8) and cryotop (77.3±8.5 and 2.1±0.9).
Effect of vitrification containers on the developmental competence and apoptosis rate of cleavage-stage mouse embryos after vitrification and warming
In the second series of experiments, the efficiency of vitrification containers was compared by using expanded mouse blastocysts. There was no significant difference in the re-expansion rates among the EM grid (80.5%), TPS (92.0%), and cryotop (92.6%) methods. The survival rates observed using the TPS, cryotop, and EM grid were significantly different (100% vs. 96.9% vs. 93.4%). The hatching rates of blastocysts following vitrification-warming on TPS (100%) were significantly higher than those following vitrification-warming on the EM grid (87.6%) and cryotop (94.7%), which were significant at the p<0.001 level. On the other hand, the total cell numbers of the blastocysts increased significantly in the vitrified-warmed embryos on the TPS (92.3±10.8) and cryotop (95.7±10.2) container compared with the EM grid (80.4±8.9). The rate of apoptotic-positive cells in the blastocysts decreased significantly in the vitrified-warmed embryos on the TPS (3.1±0.9) and cryotop (5.1±1.3) container compared with the EM grid (8.3±2.9) at the p<0.001 level.
In the third series of experiments, the efficiency of the vitrification containers was compared using mouse hatching blastocysts. The survival and re-expansion rates for hatching mouse embryos cryopreserved by the EM grid and TPS methods are shown in Table 2. There was no significant difference in the re-expansion rates and hatched rates between the EM grid (92.7% and 60.4%) and TPS (92.5% and 75.5%) methods. However, the survival rates of embryos using the TPS (100%) were higher than those using the EM grid (92.2%) method (p<0.05).
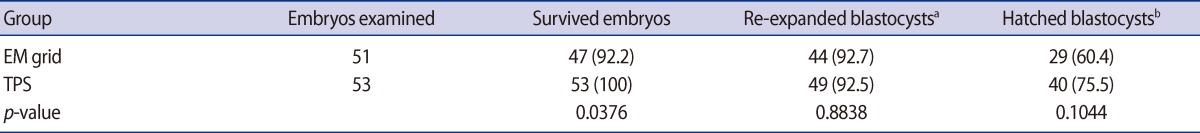
Effect of vitrification containers on the developmental competence of hatching-stage mouse blastocysts after vitrification and warming (chi-square test)
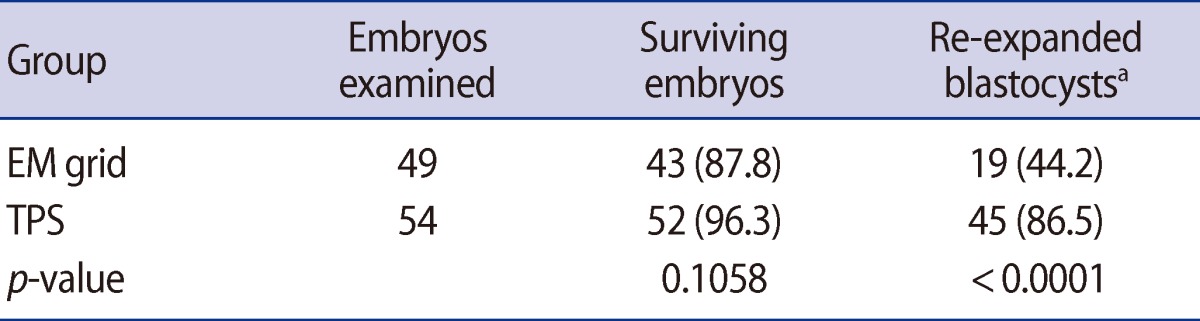
In the fourth series of experiments, the efficiency of the vitrification containers was compared using mouse hatched blastocysts. The survival rates for hatched mouse embryos cryopreserved by the EM grid and TPS methods are shown in Table 3. There was no significant difference in survival rates between the EM grid and TPS methods (87.8% vs. 96.3%, respectively). However, the re-expanded rates following the TPS method were higher than with the EM grid method (86.5% vs. 44.2%, respectively; p<0.05).
Discussion
Vitrification of mouse embryos has been performed in a variety of vitrification containers using the EM grid [33,34], cryotop [22,35,36], cryoloop [15], and OPS [10-12] methods. In this study, to develop a simple, rapid, cost-effective, and successful material for simultaneous vitrification, we investigated the effects of cleavage-stage mouse embryos and blastocysts during vitrification using several types of containers. The new container, TPS, displayed advantages that included loading on a surface prepared from commonly available non-slippery material, ease of portability with a grip, and ability to directly plunge into LN2 for storage. The post-warming developmental ability of embryos vitrified-warmed on TPS was comparable to those vitrified-warmed on the EM grid and cryotop, and was effective as judged by the apoptosis index score.
The major damaging factors that occur during cryopreservation include chilling injury, osmotic stress, cryoprotectant toxicity, and ice crystallization [28-30]. Kuwayama et al. [37] reported that use of a cryotop container improves the survival rate of vitrified-warmed embryos and has advantages over conventional vitrification procedures, in that the small volume (<1 µL) results in both rapid and uniform heat exchange during cooling. The rapid cooling rate that is also obtained with the cryotop prevents chilling injury to sensitive cells [20]. Son et al. [4] reported the use of an EM grid for vitrification of human embryos. The EM grid was originally designed for vitrification of exceedingly chill-sensitive Drosophila embryos [3]. Characteristically, the EM grid has an approximately 3-fold higher cooling rate than that obtained with the OPS container. Also, the increased rate of cooling could decrease the chilling injury of embryos during vitrification [3,38]. Based on these reports, we suggested that the synergistic effect of the TPS container may allow easy and rapid vitrification in commonly available non-narrow materials and by direct plunging into LN2 for storage. An additional advantage of the TPS container is that it effectively reduces ice crystal formation because of the enhanced cooling rate. In our study, a significantly higher developmental ability of expanded, hatching-stage, and hatched-stage blastocysts that were vitrified-warmed on the TPS container, and survived, re-expanded, and hatched-stage after culture, was observed compared to those that were vitrified-warmed on the EM grid container tested in this study (p<0.05) (Tables 2-4). On the other hand, contrary to our expectations, the developmental rates of vitrified-warmed cleavage-stage embryos using a TPS container were similar to those vitrified-warmed on EM grid and cryotop containers (Table 1). However, it provides an open container system vitrification wherein embryos come into direct contact with LN2. Thus, one fault in the use of the TPS container could be possible safety issues arising from contaminated LN2 or infected embryos during storage. Therefore, for its use in humans, further studies are required to screen for microbial infection and store embryos separately after vitrification using TPS as a container [39]. Therefore, we suggest that the major concerns needing to be addressed by users in considering carrier devices are the holding capacity, aseptic stringency, convenience of handling, and economic issues.
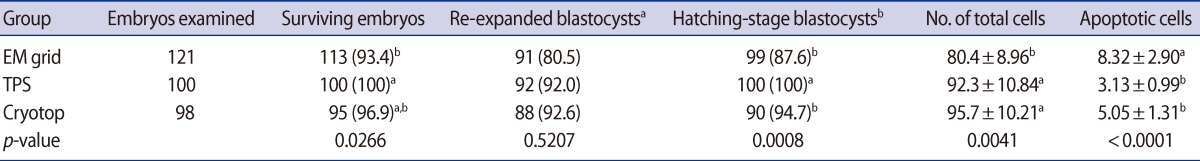
Effect of vitrification containers on the developmental competence and apoptosis rate of mouse blastocysts after vitrification and warming
A limitation of this work was the use of vitrified-warmed mouse embryos. Monitoring of outcomes with vitrified human embryos that have been transported between clinics is imperative to fully appreciate the risks associated with new vitrification containers. Therefore, in this study, we confirmed the apoptosis rate after vitrification using different containers and at different cell stages. Apoptotic cell death occurs in preimplantation mammalian embryos due to stresses, which in turn decreases the developmental competence of mouse embryos [38,40,41]. Thus, the apoptotic pattern of mouse embryos should be considered. In this study, the total number of cells and apoptosis rates by the DAPI and TUNEL methods was observed in mouse embryos after vitrified-warming derived from cleavage and blastocyst stage embryos using EM grid, cryotop, and TPS. The total number of cells and apoptosis rate of cleavage-stage mouse embryos using the TPS container was similar to those obtained with the EM grid and cryotop. However, the cryotop and TPS groups displayed higher total cell numbers and lower apoptosis rates in vitrified-warmed blastocysts (p<0.05) (Table 4, Figure 3). These data indicate that the TPS method may be effective for minimized chilling damage, a short time of exposure to toxic chemicals, and elimination of fracture damage.

The fluorescent image patterns of total and apoptotic cells in mouse embryos. (A-C; ×400) Total cells were determined by DAPI (blue), (D-F; ×400) apoptotic cells were confirmed by TUNEL (green), and (G-I; ×400) colocalization with DAPI is indicated as blue-green. DAPI, 4', 6-diamidino-2-phenylindole; TUNEL, terminal deoxynucleotidyl transferase (TDT) mediated dUTP nick end labeling; EM, electron microscopy; TPS, thin plastic strip.
In conclusion, the TPS method is simple, rapid and inexpensive, and the handling and storage of vitrified embryos can be based on the existing tools and methods of vitrification. The TPS method may also be suitable for vitrification of embryos at different stages of development.
Notes
No potential conflict of interest relevant to this article was reported.