Does blastomere biopsy in preimplantation genetic diagnosis affect early serum β-hCG levels?
Article information
Abstract
Objective
To determine whether the serum β-human chorionic gonadotropin (hCG) profile following preimplantation genetic diagnosis (PGD) is lower than that of intracytoplasmic sperm injection (ICSI) cycles.
Methods
A total of 129 PGD cycles and 1,161 age-matched ICSI cycles, which resulted in pregnancy (serum β-hCG≥5 mIU/mL) on post-ovulation day (POD) 12 were included. We compared the mean serum β-hCG levels on POD 12, 14, 21, and 28, doubling time of serum hCG, and created a cut-off value for predicting a singleton pregnancy in each group.
Results
The mean serum β-hCG concentration of the PGD group was significantly lower than that of the control group on POD 12, 14, and 21. The doubling time of serum β-hCG at each time interval showed no significant difference. The cut-off-value of serum β-hCG for predicting a single viable pregnancy was 32.5 mIU/mL on POD 12 and 113.5 mIU/mL on POD 14 for the PGD group, which was lower than that for the control group.
Conclusion
Blastomere biopsy may decrease the β-hCG-producing activity of the trophoblasts, especially in early pregnancy. Setting a lower cut-off value of serum β-hCG for predicting pregnancy outcomes in PGD may be needed.
Introduction
Human chorionic gonadotropin (hCG) can be detected in the maternal serum as early as eight days after conception. Serum β-hCG can be detected in 5% of cycles 8 days after the preovulatory luteinizing hormone surge, in 16% after 9 days, in 53% after 10 days, and in 100% of all cycles after day 11 [1]. During embryo development, β-hCG mRNAs have been identified in a single blastomere in a 4-cell stage embryo, but not in a 2-cell stage embryo [2]. In human blastocysts, β-hCG mRNA is differentially expressed mainly in trophectoderm cells, with very little or none present in the inner cell mass (ICM) [3]. Produced and secreted by syncytiotrophoblasts, its serum level represents the trophoblastic mass [4]. The amount of β-hCG in culture media secreted by the blastocysts after biopsy is inversely proportional to the number of trophectoderm cells removed [5]. However, there are no studies on serum β-hCG levels in early pregnancy after a preimplantation genetic diagnosis (PGD) procedure, especially after blastomere biopsy. Removing one or two blastomeres from the eight cell stage embryo is one of the techniques of PGD and, after this procedure, according to one study, the total number of cells and the number of both trophectoderm (TE) and ICM cells of the biopsied embryos at the blastocyst stage were reduced, although the ratio of ICM to TE cells was maintained in both 7/8 and 6/8 biopsied embryos [6].
It is beneficial for the patient, her family, and the clinician to have a reliable prognostic marker of pregnancy outcomes at the early stage. A single early serum β-hCG test or serial serum β-hCG determinations have been used to monitor early pregnancy outcomes, with serial measurements known to have greater predictive accuracy [7]. Low levels of serum β-hCG in early pregnancy have been reported as a predictor of poor pregnancy outcomes, including chemical pregnancy, ectopic pregnancy, and miscarriages [7-10]. Undergoing IVF until pregnancy is confirmed can be very stressful, but PGD can be even more stressful because of the fear of an abnormal pregnancy or repeated pregnancy loss.
Decreased trophectoderm cell numbers after biopsy may influence the secretion of β-hCG from the syncytiotrophoblasts and the implantation potential in early placentation. Thus, we hypothesized that the serum β-hCG profile could be adversely affected after blastomere biopsy in PGD cycles. The aim of this study was to evaluate whether blastomere biopsies affect early serum β-hCG concentration in pregnancies following PGD. In addition, we attempted to determine if a different cut-off value of serum β-hCG in PGD is needed for prediction of a successful pregnancy.
Methods
1. Study design and patients
The study was designed as a retrospective age-matched case-control study. The records of all patients of the IVF Clinic of Cheil General Hospital during January 2000 to May 2006 were retrospectively reviewed. This study was approved by our institutional review board.
One hundred twenty-nine pregnancies achieved by PGD with an initial serum β-hCG concentration≥5 mIU/mL on post-ovulation day (POD) 12 were recruited. The main indications of PGD were structural chromosomal abnormalities: reciprocal translocation (63.6%), Robertsonian translocation (14.0%), and inversion (4.7%). Other indications were single gene disorder (7.0%), aneuploidy screening (7.8%), and sex chromosomal abnormality. A randomly selected group of 1,161 age-matched patients who underwent intracytoplasmic sperm injection (ICSI) during the same period with an initial serum β-hCG concentration≥5 mIU/mL POD were selected as the control group. The most frequent indications for the control group were male factor (35.1%), tubal factor (22.6%), and endometriosis (18.0%). The other indications included ovulatory factor (7.9%), and advanced maternal age (1.1%), as well as unexplained reasons (9.1%). The reasons the patients in this study underwent either procedure are summarized in Table 1.
We excluded those patients with factors that may contribute to early implantation, such as azoospermia, adenomyosis, uterine anomaly, uterine synechiae, and infection. Pregnancies resulting from cryopreservation and donor transfer were also excluded.
2. Treatment protocols and clinical procedures
Ovarian stimulation was performed using a GnRH agonist or antagonist, recombinant FSH, or hMG. Following ovarian stimulation, follicles were aspirated and fertilized by ICSI. Fertilized oocytes were cultured in G1.5 medium (Vitrolife, Kungsbacka, Sweden) for 3 days. For the PGD group, on the third day, the embryos were transferred to the biopsy medium (MediCult, Jyllinge, Denmark). Acidified Tyrode's solution (pH 2.3-2.4) was applied to create a small hole in the zona pellucida. One or two blastomeres were biopsied by gentle aspiration with a polished micropipette. Zona drilling and blastomere biopsy were performed using a single pipette with a 30-µm inner diameter. The embryos were then repeatedly washed, transferred to G2.5 medium, and cultured under 6% CO2 in air at 37℃. Embryos with normal or balanced FISH signals, or that were unaffected after PCR, were selected and transferred on the fourth or fifth day of culture. The embryo transfer for the control group was performed mainly on day 3 (range, 2-5 day). Serum β-hCG levels were measured by radioimmunoassay on POD 12, 14, 21, and 28. The sensitivity of the assay was 3 mIU/mL, with intra-and interassay coefficients of variation of 1.9% and 2.5%, respectively.
3. Pregnancy outcomes
Clinical pregnancy was defined as the presence of a fetal heart beat using ultrasonography at approximately 6 to 7 weeks. Amniocentesis was performed to confirm the results of PGD in clinical pregnancies and miscarriages when samples were available.
The doubling time of serum β-hCG was defined as the number of days required for the serum β-hCG concentration to double and was calculated from the following formula:
Doubling time (DT)=(Log2)×(Time interval in days)/Log (hCG2/hCG1).
A viable pregnancy was defined as an ongoing pregnancy beyond 20 weeks of gestation or delivery from a singleton pregnancy (where one baby was born, including stillbirths, from a pregnancy in which only one gestational sac and one fetal heart beat had been demonstrated). Non-viable pregnancies included clinical abortions and ectopic and biochemical pregnancies.
4. Statistical analysis
Each serum β-hCG level was analyzed after log transformation because of non-normal distribution. We compared the mean serum β-hCG levels and doubling times in cycles of a single gestational sac which resulted in delivery (including stillbirth) because of the variation of β-hCG values in multiple pregnancies. All analyses were conducted using the SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). A chi-square test or t-test was used for statistical analysis and p-values<0.05 were considered significant. To determine the probable cut-off limits for serum β-hCG values for predicting successful pregnancy outcomes in both groups, receiver operating characteristic (ROC) curves were generated.
Results
One hundred twenty-nine pregnancies achieved by PGD with an initial serum β-hCG concentration ≥5 mIU/mL on POD 12 had structural chromosomal abnormalities. The mean age of both groups was 31.7 years (range, 25.0-42.1 years), The mean number of transferred embryos in the PGD group was 2.7±1.0, which was significantly lower than that of the control group (3.6±1.0). When outcomes were examined in pregnancies established by PGD or in the control group, the biochemical pregnancy rate, clinical abortion rate, and ectopic pregnancy rate showed no significant differences. Also, neonatal outcomes, including infant weight and prematurity rates, were comparable. However, the multiple pregnancy rate was significantly lower in the PGD group (OR, 0.551; 95% confidence interval [CI], 0.318-0.953; p=0.031) (Table 2).
Figure 1 shows the regression line of the serum β-hCG profile during early pregnancy in cycles of a single gestational sac which resulted in delivery in both groups. Both lines are horizontal with a lower level for the PGD group (95% CI of the regression line) and the lines do not cross, which indicates the serum β-hCG level is consistently lower in the PGD group in early pregnancy.
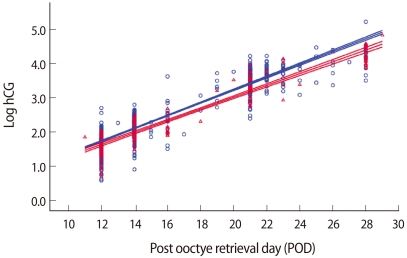
The regression lines and 95% confidence intervals of β-hCG values in early pregnancy in women who later had viable singleton pregnancy and delivery after control and preimplantation genetic diagnosis (PGD) group. The β-hCG values were log transformed before regression analysis. Red line, PGD group; Blue line, control group.
In examining the doubling of serum levels at each time interval, the PGD group showed doubling at 1.31, 1.63, and 2.53 days, which was not significantly different from the control group (Table 3). The mean serum β-hCG levels in cycles which resulted in a single gestational sac and singleton delivery are described in Figure 2. The levels of serum β-hCG on POD 12, 14, and 21 were 37.9 mIU/mL, 111.3 mIU/mL, and 2,225.0 mIU/mL in the PGD group and 46.9 mIU/mL, 145.4 mIU/mL, and 3,614 mIU/mL in the control group, and the differences were significant (p=0.023, 0.006, 0.000, respectively). However, the mean serum β-hCG levels on POD 28 showed no difference (p=not significant).
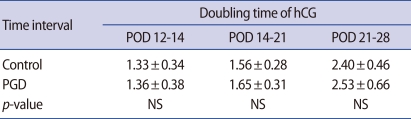
The doubling times (in days) of human chorionic gonadotrophin (hCG) secretion for each phase of the pregnancy cycles which resulted in a single gestational sac and singleton delivery
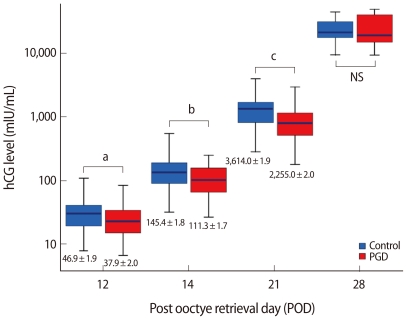
The highest and lowest quartile mean values of serum human chorionic gonadotrophin (hCG) on POD 12, 14, 21, and 28 in the preimplantation genetic diagnosis (PGD) and control groups. Values are presented as mean±SD. NS, not significant.
ap=0.023, bp=0.006, cp=0.000.
ROC curves for the mean serum β-hCG levels in cycles which resulted in a single gestational sac and singleton delivery were used to determine the points of inflection (specificity and sensitivity) to predict potential viability. From the ROC curves shown in Figure 3, the POD 14 curve is steeper and deviates to the left than the POD 12 curve, which implies that β-hCG concentration on POD 14 is a better predictor of fetal viability than β-hCG concentration on POD 12. On POD 12, the control group with a cut-off level of 40.5 mIU/mL showed a sensitivity of 79.6% and specificity of 76.9%. The positive predictive value (PPV) was 89.9%, while the negative predictive value (NPV) was 59.1%. With similar sensitivity and specificity, the cut-off value of the PGD group was 32.5 mIU/mL, which was lower than that of the control group (sensitivity 70.4%, specificity 70.8%, PPV 80.2%, NPV 58.6%). On POD 14, the control group, with a cut-off level of serum β-hCG of 113.5 mIU/mL, had a sensitivity of 81.3% and a specificity of 78.7%. The PPV was 91.9%, while the NPV was 60.0%. With similar sensitivity and specificity, the cut-off value of the PGD group was about 77.0 mIU/mL, which was lower than that of the control group (sensitivity 79.7%, specificity 77.3%, PPV 85.5%, NPV 69.4%). If we apply the same cut-off value as in the control group for PGD on POD 14, the sensitivity and NPV are only 66.2% and 59.1%. After applying the new cut-off value for the PGD group, the sensitivity and NPV increase to 79.3% and 69.4%, respectively.
Discussion
To the best of our knowledge, no study thus far has dealt with the correlation of serum β-hCG profiles in early pregnancy for PGD cycles. We analyzed the mean serum β-hCG levels in cycles which resulted in a single gestational sac and singleton delivery and suggest that serum β-hCG in early pregnancies of a PGD group is lower than that in a control group.
We measured the serum β-hCG level from post-oocyte retrieval day 12. It would be more reasonable to compare the serum β-hCG level measured based on the oocyte retrieval day than the embryo transfer day, because the oocyte retrieval day is the day of initiating fertilization. The actual amount of β-hCG is thought to reflect the number of viable trophectoderm cells producing the hormone [11]. Initially low β-hCG levels and a consistently long doubling time may reflect abnormal implantation, poor trophoblastic activity, or both [12]. Pregnancies with low initial β-hCG levels have previously been shown to have a poor prognosis in a multitude of studies [8-10,13]. In this study, although the initial serum β-hCG levels were low in the PGD group, the biochemical pregnancy rate and miscarriage rate were similar to those of the control group. This may indicate that once pregnancy is initiated, any adverse impact of PGD is undetectable, and that low serum β-hCG levels in PGD are not a strong predictor of an abnormal pregnancy outcome. Additionally, there were no significant differences in second trimester losses, preterm delivery rates, or birth weights between the groups. In brief, the serum β-hCG concentration was lower in the PGD group, but the overall pregnancy outcomes of the two groups were quite similar.
The doubling time of β-hCG in normal pregnancies is 1.4-3.5 days during the first 20-30 days of gestation [14]. Abnormal pregnancies often have a normal doubling time early in their course and subsequently become aberrant. Our data indicate that the doubling times of both groups were in the normal gestational growth range until the 35th day of gestation. Since a low doubling time may reflect abnormal implantation or poor trophoblastic activity, this data may indicate that trophoblastic activity was not reduced in PGD cycles.
If the biopsied blastomere is destined to be the trophectoderm, the biopsy procedure may lead to a reduction in trophoblastic cell mass. Based on the fact that the amount of β-hCG secreted by the blastocyst after biopsy was inversely proportional to the number of trophectoderm cells removed [5], blastomere biopsy may lead to decreased serum β-hCG production in early pregnancy.
In PGD, the genetic diagnosis is made by the analysis of one or two blastomere biopsies. Apart from the reduction in cellular mass, Hardy et al. found that the removal of one or two cells at the 8-cell stage does not adversely affect the preimplantation development of biopsied embryos in vitro [6]. Also, there was no significant difference in the number of cells in blastocysts, trophectoderm, or ICM depending on whether one or two blastomeres were biopsied [15]. Blastomeres can regain the losses from a biopsy, but we did not analyze the number of cells at the moment of embryo transfer, which may be the one of the limitations of our study.
In our study, the PGD group produced lower β-hCG levels on tests at POD 12, 14, and 21 compared to the control group. A few possible mechanisms might explain the discrepancy observed at the time of each blood test. First, the observed discrepancy may have resulted from a difference in the rate of very early subclinical losses, since the average number of embryos in each transfer was greater for the control group than for the PGD group. Second, a different embryo transfer day may result in a gender bias, which in turn has been suggested to be related to the rate at which the early embryo develops. To adjust for the difference of embryo transfer dates, outcomes were examined in pregnancies established by day 3 or day 5 transfers, and we found that the biochemical pregnancy rate and miscarriage rate were similar between the two groups (data not shown). Thus, the variability of ET days does not appear to significantly influence the range of β-hCG, as evaluation of the data when grouped by transfer day did not show significant differences. The results of the comparison of the mean β-hCG values between the day 5 and day 3 transfer groups are controversial. Papagerorgiou et al. [16] showed that there were no differences in the mean β-hCG levels between the two groups. In contrast, Zhang et al. [17] showed that initial β-hCG levels in pregnancies resulting from day 5 transfers were lower than those from day 3 transfers. These differences may have resulted from other mechanisms than the blastomere biopsy itself, and larger studies may be needed to confirm these controversial results. Third, the blastomere biopsy procedure in the PGD cycle might directly and adversely affect the trophectoderm cell mass in the development of the embryo, resulting in a subtle delay or reduction in β-hCG production.
In conclusion, our results suggest that PGD may adversely affect serum β-hCG levels in early pregnancy. Setting a lower cut-off value for predicting a successful pregnancy in PGD could be helpful in counseling pregnant patients following PGD.
Notes
Presented in part at the 63rd Annual Meeting of the American Society for Reproductive Medicine, Washington DC, USA, 13 October to 17 October, 2007.
No potential conflict of interest relevant to this article was reported.


