Developing a deeper insight into reproductive biomarkers
Article information
Abstract
The development of biomarkers of reproductive medicine is still in its infancy because many black boxes are still present in reproductive medicine. Novel approaches to human infertility diagnostics and treatment must be developed because reproductive medicine has lagged behind in the implementation of biomarkers in clinical medicine. Despite the dearth of the available literature, the current rapid pace of publications suggests that this gap will soon be filled therefore; this review is a précis of the research that has been done so far and will provide a basis for the development of biomarkers in reproductive medicine.
Introduction
Any biological index with the potential to be measured that indicates a defined biological endpoint, such as a disease or developmental stage, is known as a biomarker. Biomarkers may include a wide range of measurable, significant targets, including cellular, biochemical, immunological, genetic, physiological, and molecular changes. Biomarkers help us acquire knowledge about the effects and nature of an exposure and the vulnerability of organisms towards the noxious impacts of that exposure.
Large-scale research has been conducted into the development of biomarkers to predict patients' responses to drugs and to diagnose clinical conditions, extending to all therapeutic areas, including reproductive medicine. Biomarkers can be used to identify subgroups for whom treatment is likely to be successful, to improve the assessment of exposures, and to predict the results, consequences, and classification of subgroups of potentially diverse disease etiologies [1]. It has been reported that 1.5 million women are infertile [2]; likewise, another study documented a prevalence of infertility of 9% among males [3].
Biomarkers can serve as a measurable indicator of proliferation and differentiation indices [4], the formation of DNA adducts or damage [5], apoptotic endpoints [6], chromosomal abnormalities [7], measurements of enzyme activity [8], micronucleus formation [9], changes in gene expression profiles [10], the expression of specific genes [11], and the presence of a parent compound or metabolite [12].
Biomarkers associated with reproductive impairment are principal early-warning signals of ecosystem impacts, and they require complete validation and characterization in an ecosystem [13]. Potential biomarkers of reproductive impairment are in various stages of development.
Biomarkers serve several distinct purposes in reproductive medicine, including: (1) biomarkers of a disease or developmental stage: some biomarkers serve as indicators of specific phases in normal and abnormal developmental processes; (2) biomarkers of effect: physical or chemical environmental exposures can generate an assembly of localized or systemic effects and can be measured at the molecular, cellular, or clinical level; (3) biomarkers of exposure: biomarkers can be used to identify potential toxic exposures because of significant changes in biological function or appearance that signify exposure to a specific stimulus of a biological, physical, or chemical nature; (4) biomarkers of susceptibility: genetic biomarkers may also be used to identify predisposition to develop specific conditions [14].
Improving exposure assessments refers to differentiating subfertile patients from infertile patients and identifying the severity and extent of subfertility. A marker can also identify when a couple is best served by in vitro fertilization. Monitoring susceptibility to the effects of treatment and identifying subgroups may help individualize treatment of patients who are in dire need of a higher dose of medication or alteration of standard laboratory conditions such as oxygen tension or media. The accuracy of the prognosis may be improved by early detection of the number of embryos or which embryo is to be used in in vitro fertilization and the prediction of abortion, obstetric complications (preterm labor or preeclampsia), and ectopic pregnancy. The classification of patients into subtypes with different etiologies helps differentiate between implantation failure and unexplained infertility.
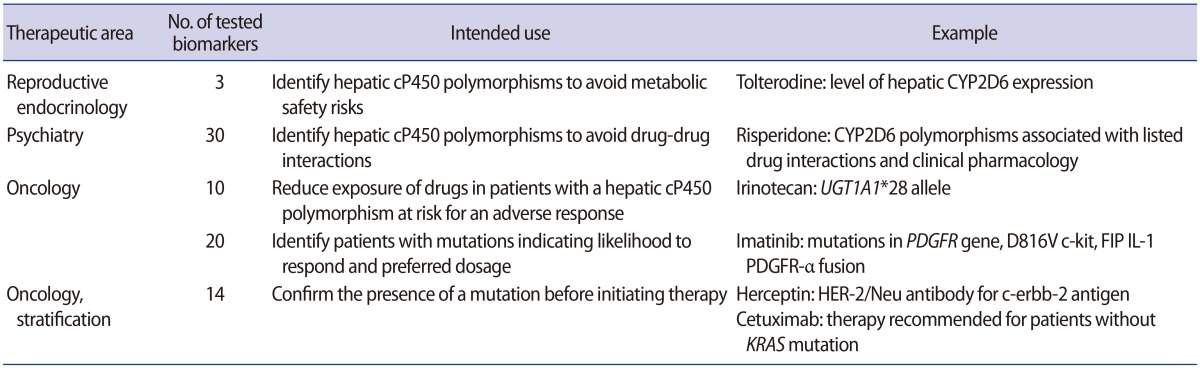
Most reproductive biomarkers that have been developed so far deal with diagnostics, such as the diagnosis of polycystic ovarian disease, endometriosis, infertility, and the viability and location of early pregnancy. Multivariate biomarkers have also been developed; for example, the first diagnostic test based on a multivariate biomarker was OVA1, developed by Vermillion intended to be used for debulking surgery of patients suffering from ovarian adnexal masses. Recently, another multivariate biomarker to diagnose endometriosis has also been developed. To date, about 20 biomarkers have been identified in the serum for the early diagnosis of ectopic pregnancy [15]. Biomarkers approved by the Food and Drug Administration are shown in Table 1.
Neohormones as reproductive biomarkers
Neohormones are new paracrine or endocrine adaptations that have the ability to define mammalian success to complement physiological functions. Relaxin like peptide hormones are common neohormones, as they define basic reproductive physiology, such as viviparity with placentation or implantation, lactation, and adaptations required by sperm cells for successful internal fertilization, thereby acting as highly useful biomarkers to characterize and monitor reproductive diseases. H2-relaxin assists in implantation and the development of the placenta in the ovary, whereas its levels change in cases of early miscarriage. During fetal development, testicular INSL3 is important for the first phase of testicular descent, but it may be disrupted in cryptorchidism. In adults, INSL3 is considered to be an antiapoptotic factor in follicle selection (female) and germ cell survival (male), and serves as an excellent indicator of Leydig cell functional capacity, especially in aging males. Likewise, INSL5 and INSL6 have been reported to be involved in the maintenance of adequate spermatogenesis [16].
Endocannabinoids as potential biomarkers in reproductive medicine
Endocannabinoids and anandamide have proven to be potential biomarkers of reproductive impairments. These are a group of bioactive lipids that act as crucial signals in human reproduction. Fluctuations in the balance between the degradation or decay and synthesis of endocannabinoids lead to local changes in the human male and female reproductive tracts, which in turn adjust and control several pathophysiological processes, including sperm and oocyte maturation [17]. A study revealed that endocannabinoids measured in the saliva could be used as a biomarker of obesity [18].
Biomarkers of common reproductive impairments in males
About 5%–20% of males suffer from infertility because of obstructive azoospermia, non-obstructive azoospermia, and pretesticular azoospermia. A study found that several proteins expressed in the epididymis and testis were directly associated with fertility. The concentration of seminal plasma proteins such as TEX101 and ECM1 can be used as biomarkers for diagnosing azoospermia [19]. The down regulation of several genes, such as SPATA3, SPACA4, FAM71F1, UBQLN3, GGN, and AKAP4 has been observed in infertile males. Likewise, the upregulation of TMEM225, ADCY10, WBSCR28, GSG1, FSCN3, GTSF1L, and SPATS1 was identified in males with late maturation arrest. Therefore, a molecular diagnostic tool for determining the degree of spermatogenic impairment could be developed using those genes [202122]. Another study [23] found that total measurements of serum cathepsin D and K activity and total sialic acid can serve as biomarkers for distinguishing between benign prostatic hyperplasia and prostate cancer.
The DNA degradation index has been proven to be a noninvasive biomarker for identifying infertile individuals with varicocele, a condition that causes impaired testicular function and is associated with increased fragmentation in sperm DNA [24]. Seminal plasma can be used in the discovery of biomarkers because the seminal plasma proteome contains a plethora of proteins, including tissue-specific proteins, and may be used to monitor pathological processes [25].
Biomarkers of common reproductive impairments in females
About 15% of the female population faces infertility issues because of premature ovarian failure [26]. Anti-Müllerian hormone has been proven to be a sensitive biomarker for detecting the menopausal transition [27]. Another study [28] suggested that low serum anti-Müllerian hormone levels are an indication of early ovarian aging. The level of serum miR-21 is elevated in women suffering from polycystic ovary syndrome, so serum miR-21 can act as a biomarker for diagnosis of polycystic ovary syndrome [29]. A study [30] reported that the measurement of adipokine levels in serum is not a good marker for polycystic ovary syndrome, whereas high levels of anti-Müllerian hormone and low sex hormone-binding globulin levels indicate polycystic ovary syndrome. Oncofetal protein IMP3 may act as a diagnostic biomarker for the detection of endometrial cancers. An increase in the expression of IMP3 has been observed in the decidualized endometrial stroma of chorionic villi and gestational endometrium during early pregnancy [31]. The hypermethylation of SOX1, HS3ST2, and AJAP1 is an indicator of endometrial cancer in atypical hyperplasia patients. Therefore, these genes can also be used as biomarkers for screening atypical hyperplasia [32].
Biomarkers of an abnormal corpus luteum
1. Progesterone
Progesterone is a pregnancy hormone, and its presence in the blood was confirmed in 1958. Its level rises from less than 0.1 µg/100 mL (nonpregnant) up to 87 µg/100 mL during pregnancy [33]. Low progesterone levels have been reported in cases of premature births. Progesterone keeps the uterus quiet during pregnancy, reduces the mother's immune response, and supports the implantation of the fetus in the uterus. A decrease in progesterone levels facilitates the onset of labor and stimulates milk production. Lower progesterone concentrations than normal are the leading cause of premature labor [3435], and this can be prevented by administering progesterone to pregnant women [3637].
Serum progesterone has been reported as a potential biomarker for differentiating nonviable pregnancies from viable pregnancies [3839]. The combination of human chorionic gonadotropin with progesterone improves the specificity of monitoring fetal viability during early gestation [38]. Receptors of estrogen and progesterone act as prognostic biomarkers of ovarian cancer [40].
2. Inhibin
An ovarian hormone known as inhibin inhibits the secretion of follicle-stimulating hormone. This hormone regulates fertility and declines to a negligible level after menopause. It has been reported that some life-threatening ovarian malignancies, such as granulosa cell tumors and mucinous carcinomas, continue to secrete inhibin into the serum. Therefore, a highly accurate diagnostic test based on serum inhibin levels has been developed for granulosa cell tumors and mucinous carcinomas. Likewise, when the cancer antigen 125 (CA-125) test is used in conjunction with an inhibin assay, epithelial ovarian carcinomas are detected, and both of these tests can also identify a large majority of ovarian cancers with high specificity and sensitivity [41]. It has been reported in the New England Journal of Medicine that the serum concentration of inhibin was elevated in most postmenopausal women with granulosa cell tumors and mucinous carcinomas, but a significant decrease in the concentration was observed after removal of the tumor [42].
Inhibins act as biomarkers of reproductive cancers. In various prostate and gonadal cancers, the concentration of inhibin A and inhibin B is elevated, so they act as diagnostic biomarkers for reproductive cancers and as immunocytochemical markers for the analysis of tissue sections [43]. Another study suggested that human sex cord stromal tumors secrete inhibin A and inhibin B, which are potent biomarkers of tumors [44].
Sertoli cells produce glycoprotein inhibin B, which plays a key role in the regulation of follicle-stimulating hormone by a negative feedback loop. The concentration of systemic inhibin B is an indicator of spermatogenic status because a higher concentration of inhibin B indicates normal fertility, whereas a lower level of inhibin B is observed in men with damaged testes because of germ cell depletion. Therefore, inhibin B has been identified as a potential biomarker for monitoring male infertility; in fact, a study reported that inhibin B combined with follicle-stimulating hormone exhibited a higher positive predictive value than inhibin for detection of male infertility [4546].
3. Renin and relaxin
Relaxin is a hormone that is proteinaceous in nature and is secreted by the corpus luteum during gestation [47]. Its concentration increases soon after conception and remains steady through the 15th week of gestation [48]. A preliminary assessment demonstrated that the levels of relaxin in the serum of individuals with a tubal ectopic pregnancy were considerably lower than in patients with a viable intrauterine pregnancy [49]. It has been proposed that renin may serve as marker of future cardiovascular events [50]. Most patients with a tubal ectopic pregnancy exhibited a low level of relaxin, so relaxin may act as a biomarker of ectopic pregnancy [51]. A study [52] reported reduced rennin levels in women with ectopic pregnancy, in comparison to women with a spontaneous miscarriage or ongoing intrauterine pregnancy.
Biomarkers of the fallopian tube and pregnancy maintenance
1. Vascular endothelial growth factor
Vascular endothelial growth factor (VEGF) is a dominant angiogenic factor important in vascular growth, remodeling, and permeability [53]. It regulates angiogenesis in the endometrium [54] and corpus luteum [55]. VEGF also plays a key role in placentation and implantation. The expression of VEGF is triggered by tissue hypoxia [56]. It was hypothesized that serum VEGF levels would be elevated in tubal ectopic pregnancy, regardless of its role in endometrial and luteal development, and it was found that VEGF levels in the serum were considerably higher in patients with a tubal ectopic pregnancy than in those with a normal intrauterine pregnancy [57]. Serum VEGF levels are usually elevated in patients with disseminated cancer [58]. The concentration of VEGF is elevated in endometriosis patients [59].
In squamous cell carcinoma of the head and neck, measurements of the serum VEGF levels may serve as a biomarker for the prognosis of response to therapy, and a study reported its use as a diagnostic biomarker of major depressive disorder [6061]. In contrast, another study suggested that nerve growth factor beta (NGF-β) and VEGF are principal neurotrophic factors of the male reproductive system. They reported that a decrease in VEGF and NGF-β was associated with increased testicular damage and apoptosis in diabetic rats. Testis NGF-β and VEGF levels may prove to be effective novel biomarkers of diabetes-induced testicular damage [62].
2. Creatine kinase
Creatine kinase is an enzyme released in response to muscle damage, and may also be used as a marker of fallopian tube damage and a biomarker for the diagnosis of myocardial infarction [63]. A study of women with ruptured ectopic pregnancies, unruptured ectopic pregnancies, and normal intrauterine pregnancies found higher concentrations of creatine kinase in the serum of individuals suffering from ruptured tubal ectopic pregnancy than in those with a normal intrauterine pregnancy or unruptured tubal ectopic pregnancy. The concentration of creatine kinase is influenced by tubal location, because it is considerably higher in women with an isthmic pregnancy than in ampullary pregnancies. Moreover, it was recently confirmed that creatine kinase levels multivariate biomarker was OVA1, higher in ruptured ectopic pregnancies and isthmic tubal ectopic pregnancies. Tubal ectopic pregnancy grows and progresses towards a state of causing damage, so the increase in the concentration of serum creatine kinase in the serum is a marker for monitoring whether a pregnancy is normal [6465].
Biomarkers of abnormal fetal growth
1. Pregnancy-specific b-1-glycoprotein (Schwangerschaft protein 1)
Pregnancy-specific beta glycoprotein or Schwangerschaft protein 1 (SP1) is one of the earliest proteins to be detected in immunohistochemical techniques and trophoblast culture as a biomarker [66], and its function in the diagnosis of ectopic pregnancy has additionally been supported and validated by proteomic discoveries [67]. When serial analyses of SP1 were assessed using an enzyme-linked immunosorbent assay in apparently normal pregnancies and complicated pregnancies, SP1 was found to decrease with increasingly severe retarded intrauterine growth, while it increased with increasingly severe hypertension during pregnancy [68].
2. Human placental lactogen
Human placental lactogen (hPL) is secreted by the placenta, and can be monitored during the first trimester of pregnancy. Levels of hPL are lower in pregnant women with tubal ectopic pregnancy than in those with a normal intrauterine pregnancy, especially after 7 weeks of gestation. The placenta synthesizes hPL that is secreted into the maternal serum [6970]. hPL serves as diagnostic biomarker to screen for Down syndrome and other impairments during the first trimester of pregnancy [71].
3. Human chorionic gonadotropin
Human chorionic gonadotropin (hCG) is the only biomarker currently used routinely in clinical practice [7273]. hCG is released by trophoblasts and an increase in its level indicates the viability and survival of an embryo. It is a heterogeneous glycoprotein, consisting of an alpha and beta subunit that can differ in carbohydrate and peptide structure. Pregnancy can be monitored easily because immediately after conceiving, early trophoblast cells start producing hCG, which acts on corpus luteum cells to promote progesterone production.
Two variants of hCG stimulate invasion, malignancy, and tumor cell growth. Cytotrophoblast cells produce hyperglycosylated hCG, which acts as a tumor marker for gestational trophoblastic diseases and as a biomarker for cytotrophoblast cells [7475]. Interactions between the secretion level of hyperglycosylated hCG and oxidative stress produced by hydrogen peroxide have been demonstrated through in vitro studies of placental function. In a study of the association between hydrogen peroxide and maternal levels of hCG, pregnant women with preeclampsia exhibited a correlation between systemic hCG levels and oxidative stress. Another study [76] demonstrated that circulating hCG could be used as a biomarker or a tool for monitoring oxidative stress during pregnancy.
4. Pregnancy-associated plasma protein-A
Pregnancy-associated plasma protein-A (PAPP-A) may act as a biomarker for the prenatal screening of trisomy 21. A study [77] identified the overexpression of PAPP-A in Down syndrome from placental messenger RNA in the mother's serum proteins. Levels of inhibin A and hCG increase in trisomy 21, whereas those of alpha-fetoprotein (α-FP) and unconjugated estriol decrease [78]. PAPP-A regulates insulin-like growth factor, which is necessary for the development of the fetus. The level of this protein increases throughout gestation, but decreases after pregnancy. Screening of Down syndrome is based on the concentration of PAPP-A measured during the first trimester of pregnancy. Levels of PAPP-A decrease during the second trimester of pregnancy in women who subsequently develop preeclampsia in comparison with those who do not. Conversely, its concentration is elevated even more in individuals with preeclampsia than those without preeclampsia during the last trimester of pregnancy [79].
5. α-Fetoprotein
α-FP acts as a diagnostic biomarker for monitoring fetal developmental abnormalities, such as Down syndrome or neural tube defects, as well as for the development of tumors such as hepatocellular carcinomas. A study of α-FP in knock-out mice revealed that α-FP is necessary for female fertility; this relationship was found to be mediated through its estrogen-binding capacity, because the anti-estrogenic action of α-FP controls female fertility [80]. α-FP is produced by the embryo and is secreted into the amniotic fluid. It may cross the placental barrier to enter the mother's blood, in which the titer is measured to diagnose developmental abnormalities of the fetus [81]. Levels of α-FP are measured at 14–22 weeks of pregnancy [82]. An elevated level of α-FP in maternal serum indicates neural tube defects such as anencephaly or spina bifida [83], and lower levels of α-FP signify a risk of Down syndrome in the developing fetus [84]. Antenatal Down syndrome is screened for using a quadruple test of α-FP, inhibin A, hCG, and unconjugated estradiol [82].
Biomarkers of normal implantation
1. Progestagen-associated endometrial protein/glycodelin (placental protein-14)
Progestagen-associated endometrial protein (placental protein-14 [PP-14]) is a glycoprotein (molecular weight: ~28 kDa) with a sequence homologous to β-lactoglobulins, consisting of a retinol-binding motif. Human serum contains progestagen-associated endometrial protein at nanomolar concentrations. This protein is secreted by the seminal vesicle epithelium in men and produced by decidualized and secretory endometrium in women [85]. PP-14 regulates the uterine environment to make it suitable for pregnancy, and also plays an important role in the occurrence and timing of the suitable sequence of events in the fertilization process [86]. In healthy women, serum PP-14 concentrations begin to increase during the mid-luteal phase and start to decline during the mid-follicular phase of the menstrual cycle, and a significant decrease in the PP-14 level is observed between the mid-follicular and mid-luteal phases of the menstrual cycle [87]. The concentration of progesterone-associated endometrial protein is lower in the endometriosis-affected endometrium than in the normal endometrium [88].
2. Leukemia inhibitory factor
Leukemia inhibitory factor belongs to the interleukin-6 family of cytokines, and it is a key contributor to human reproduction, implantation, and inflammation. It is involved in the regulation of differentiation and growth of embryonic stem cells, peripheral neurons, adipocytes, osteoblasts, endothelial cells, and primordial germ cells [89]. A study [90] revealed that leukemia inhibitory factor was associated with successful pregnancy initiation, so it may serve as a potential biomarker of pregnancy
Biomarkers of reproductive cancers
1. Follistatin
Follistatin is a glycoprotein present in the follicular fluid of the ovary. It serves as a biomarker of ovarian mucinous tumors and pregnancy. Another study suggested its use in the diagnosis of lung adenocarcinoma because lung adenocarcinoma cells secrete follistatin into the serum, which can be supportive for the survival of adenocarcinoma cells by neutralizing the action of activin A [9192].
2. Cancer antigen 125
Ovarian carcinoma is diagnosed by CA-125. It is a tumor-associated antigen released by the coelomic epithelium that serves as a useful biomarker for endometriosis [93]. It is secreted in bodily fluids in soluble form or expressed on cell surfaces that experience metaplastic differentiation into Müllerian-type epithelium. The level of CA-125 is elevated in benign conditions such as uterine fibroids and endometriosis, and its levels also increase during early pregnancy [5294]; therefore, it is a useful biomarker. Elevated levels of soluble CA-125 have been reported in several other malignant conditions, such as non-Hodgkin lymphoma [95], mesothelioma [96], leiomyosarcoma and leiomyoma of gastrointestinal origin [97], breast cancer [98], gastric cancer [99], liver diseases, ovulatory cycles [100], congestive heart failure [101102], and tuberculosis [103].
3. Interleukin-8
Interleukin-8 is an important contributing factor to male genital tract inflammation/infection. It is associated with benign prostatic hyperplasia-related inflammation. Among several chemokines and cytokines, seminal plasma interleukin-8 may serve as a predictive and reliable surrogate marker of prostatitis and leukocytospermia. It has also been reported that seminal plasma interleukin-8 is involved in the swelling of the prostate, along with other organs of the male reproductive tract, especially the epididymis and seminal vesicles, but not the testis [104]. Interleukin-8 has also been found to be a promising marker for several clinical conditions, such as non-Hodgkin lymphoma, chorioamnionitis, nosocomial bacterial infections, osteomyelitis, inflammatory bowel disease, acute pyelonephritis, pulmonary infections, vesicoureteral reflux, prostatitis, and urinary bladder cancer [105].
4. Interleukin-6
Interleukin-6 is a multifunctional cytokine that acts as a triggering factor of B-lymphocytes because it triggers the differentiation of B cells, which give rise to antibody-producing plasma cells. Interleukin-6 affects the progression of prostate cancer and prostate carcinoma, as well as the production of prostate-specific antigen [106]. Menstrual effluents contain interleukin-1β, interleukin-6, and tumor necrosis factor α, thereby acting as a biomarker of chronic endometritis [107], and it has been reported that the tumor necrosis factor α gene promoter region is more highly expressed in women with endometriosis [108].
Biomarkers for measuring the estrogenic effects of endocrine disruptors
Many environmental chemicals act as endocrine disruptors, such as dichlorodiphenyl-trichloroethane, dioxins, and polychlorinated biphenyls, which are anti-androgenic and estrogen-like in nature. They impede natural hormonal action and cause infertility in males. Prostate and testicular cancers, hypospadias, undescended testis, abnormal sexual development, Sertoli cell-only patterns, and altered thyroid and pituitary gland functions have been found to be caused by endocrine disruptors [109].
1. Complement component 3 and ornithine decarboxylase
Many genes identified in the uterus have served as marker genes to evaluate the estrogenicity of endocrine disruptors. For example, the plasma glycoprotein clusterin gene, genes responsible for gap junction connexins, such as connexin 26 and connexin 43, and complement component 3 (C3) have been shown to be controlled by the endometrium of rats. C3 was found to be produced in the uterus of female mice. C3 is a protein that is an important contributor to immunity. In the uterus, endocrine disruptors increase the expression of the gene for ornithine decarboxylase. Therefore, these genes sensitive to estrogen have served as markers to analyze the estrogenic potential of endocrine disruptors of the uterus [110111112].
2. Vitellogenin
A study done on fish has reported that vitellogenin (VTG) is a precursor protein of egg yolk secreted by liver cells and released into the blood, from which it reaches the ovaries and promotes oocyte development; moreover, it has been proposed that VTG can also be used as a potential biomarker for estrogenic chemicals [113]. Although VTG is also present in male fish, it is not normally expressed due to the low circulating level of Estradiol (E2) in the blood plasma; however, males have been shown to have ability to express VTG under the influence of estrogenic endocrine disruptors; thus, it has been proposed that the VTG gene in male fish may be used as a biomarker for analyzing the effects of endocrine disruptors [114115116].
3. pS2 and mucin 1
A study revealed that estrogenic compounds and E2 triggered the expression of pS2 in the cloning of the pS2 gene in the MCF-7 breast cancer cell line [117]. The production of pS2 mRNA can be induced by E2 in some breast cancers, but not in normal breast tissue or in any other human cell lines. Therefore, the expression of pS2 mRNA in MCF-7 cells is an ideal model for estimating the impact of estrogenic compounds [117118119]. Mucin1 is an extended rod-like molecule protruding above the surface of epithelial cells, and it acts as a well-known marker of breast cancer [120].
4. Progesterone receptor
The steroid receptor known as progesterone receptor binds with progesterone, and it is involved in a several physiological functions such as homeostasis, cell differentiation, and the control of embryonic development [121122123]. To evaluate the estrogenicity of endocrine disruptors efficiently in a cost- and time-effective manner, the expression levels of progesterone receptor genes are measured [116].
5. Calbindin-D9k
The use of calbindin-D9k (CaBP-9k) as a biomarker for endocrine disruptors has been reported. The injection of estrogenic chemicals such as nonylphenol, bisphenol A, 17β-estradiol, and 4-tert-octylphenol in immature mice led to CaBP-9k protein assembly and uterine localization, which were confirmed by western blotting and immunohistochemical staining, respectively. A time- and dose-dependent increment in the CaBP-9k protein was identified in the uterus of immature rats when treated with 4-tert-octylphenol and nonylphenol; thus, the CaBP-9k protein could serve a potent biomarker for evaluating the estrogenicity of putative estrogenic compounds [124].
Conclusion
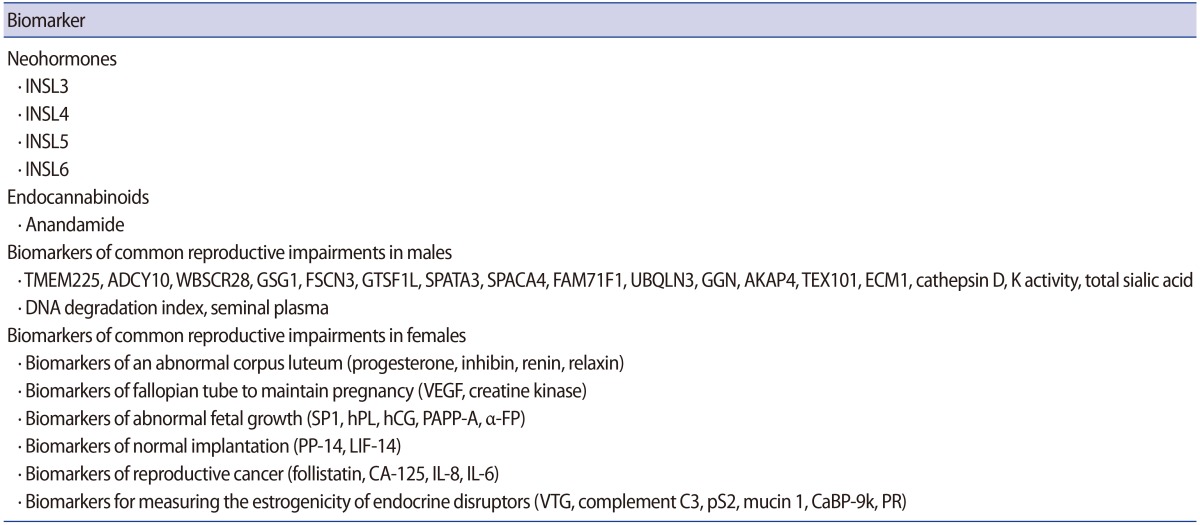
It is necessary to develop clinically useful biomarkers to inform therapeutic and regulatory decision-making bodies about candidate drugs and their effects in an attempt to resolve reproductive impairments through the development of new diagnostic methods and medicines based on biomarkers. The number of candidate markers in reproductive medicine is increasing, and it is urgently necessary to comprehend the development pathway from discovery to clinical utility. Extensive testing and modification, along with validation, must be performed before a biomarker is demonstrated to have clinical utility. New partnerships and opportunities exist and should hasten the development of reproductive biomarkers. The classification of biomarkers discussed in this review is presented in Table 2.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.