Mixed double-embryo transfer: A promising approach for patients with repeated implantation failure
Article information
Abstract
Objective
The purpose of this study was to evaluate the efficacy of frozen mixed double-embryo transfer (MDET; the simultaneous transfer of day 3 and day 5 embryos) in comparison with frozen blastocyst double-embryo transfer (BDET; transfer of two day 5 blastocysts) in patients with repeated implantation failure (RIF).
Methods
A total of 104 women with RIF who underwent frozen MDET (n=48) or BDET (n=56) with excellent-quality embryos were included in this retrospective analysis. All frozen embryo transfers were performed in natural cycles. The main outcome measures were the implantation rate, clinical pregnancy rate, multiple pregnancy rate, and miscarriage rate. These measures were compared between the patients who underwent MDET or BDET using the chi-square test or the Fisher exact test, as appropriate.
Results
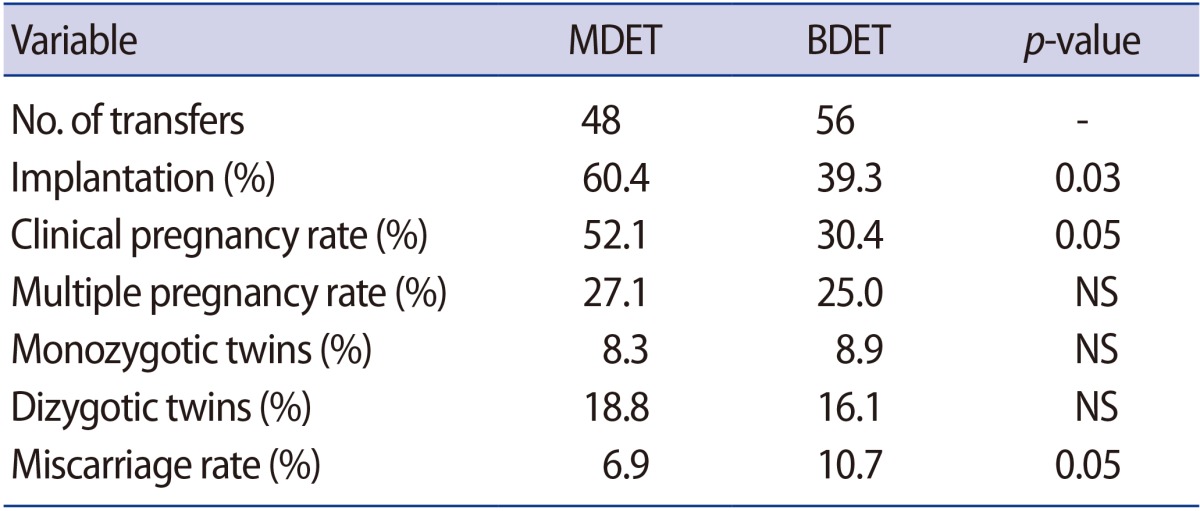
The implantation and clinical pregnancy rates were significantly higher in patients who underwent MDET than in those who underwent BDET (60.4% vs. 39.3%, p=0.03 and 52.1% vs. 30.4%, p=0.05, respectively). A significantly lower miscarriage rate was observed in the MDET group (6.9% vs. 10.7%, p=0.05). In addition, the multiple pregnancy rate was slightly, but not significantly, higher in the MDET group (27.1% vs. 25.0%).
Conclusion
MDET was found to be significantly superior to double blastocyst transfer. It could be regarded as an appropriate approach to improve in vitro fertilization success rates in RIF patients.
Introduction
The collaborative efforts of clinicians, biologists, and infertile couples in cases of repeated implantation failure (RIF) often fail to reach the desired result and successful conception. The best solution for RIF patients is to implement a strategy that includes the optimal moment for embryo transfer, as well as the appropriate developmental stage and number of the transferred embryos [12].
In determining the time of embryo transfer, we ultimately need to hit the so-called window of implantation (WOI), a relatively short period of time when the endometrium is best suited to support embryo-endometrial interactions. In humans, the endometrium becomes receptive to embryo implantation from 6 to 8 days after ovulation and remains receptive for 2 to 4 days [345]. Different timing of the WOI in at least 25% of RIF patients was confirmed based on transcriptomic modifications of the endometrium during the mid-luteal phase [6]. This is the reason why pinpointing the WOI in women with RIF is still an unresolved challenge, a quest for the mythic El Dorado.
Selecting the most suitable stage of embryo development for transfer and determining the best number of embryos are also critically important issues for achieving successful implantation. Blastocyst double-embryo transfer (BDET) tends to be the preferred practice in patients with RIF, compared to single-embryo transfer and cleavage-stage double-embryo transfer [7891011121314]. However, the results obtained with this strategy are still far from satisfactory. A possible explanation for the observed implantation failures in these patients could be the displacement of their WOI [15]. Looking for a novel solution to this problem, we decided to devise a new embryo transfer strategy, mixed double-embryo transfer (MDET). MDET combines two embryos at different developmental stages—1 cleavage stage (day 3) embryo with one blastocyst (day 5)—that are transferred together in a single frozen embryo transfer procedure in an unstimulated cycle.
We designed this study to test the hypothesis that in women with RIF, the pregnancy rate could be significantly improved by implementing MDET. MDET was compared with BDET, which is routinely used in clinical practice.
Methods
1. Study design
The present study compared the efficacy of two types of in vitro fertilization (IVF) treatment: MDET, which includes one day 3 embryo and one day 5 embryo; and BDET, the double transfer of two day 5 embryos. Both types of transfer were performed after freezing and thawing, during a natural cycle.
This retrospective study was carried out at Nadezhda Women's Health Hospital (Sofia, Bulgaria), after approval from the local Ethics Committee. The analysis included all frozen-thawed embryo transfer cycles performed between April 2015 and December 2016 that met the inclusion/exclusion criteria.
2. Patients
A total of 104 women with RIF, 48 of whom underwent MDET and 56 of whom underwent BDET, were included in the analysis. The patients who underwent frozen MDET or BDET were selected according to the following inclusion criteria: (1) women with RIF, defined as ≥3 failures of implantation in at least three consecutive IVF attempts, in which one to two high-quality embryos were transferred in each cycle; (2) women aged <42 years; (3) women undergoing oocyte retrieval after a long stimulation protocol; (4) patients with at least two excellent-quality embryos on day 3 and/or day 5 in the analysed cycle.
The exclusion criteria included major uterine abnormalities and/or pathologies, thrombophilia, known genetic disorders, as well as common contraindications for IVF and/or pregnancy as per our usual clinical practice. In addition, women with a body mass index (BMI) >30 kg/m2 or a basal follicle-stimulating hormone (FSH) level >10 IU/L were excluded from the study.
The sample size was determined by the number of participants who were eligible to be included in the study in the hospital during the allotted time period.
3. Measures
1) Baseline characteristics
The BMI was measured for each woman at the cycle scheduling visit within 2 weeks of starting treatment and calculated as weight in kilograms divided by the square of height in meters (kg/m2).
Serum FSH was measured in the early follicular phase (days 2 to 4) in the cycle preceding treatment. FSH concentrations were measured using an electrochemiluminescence immunoassay and analysed using a Cobas E411 System (Roche, Basel, Switzerland).
Ovulation and endometrial thickness were documented by transvaginal ultrasonography in the natural menstrual cycle during which embryo transfer was performed. Transvaginal ultrasonography was performed in the morning using a 6–10 MHz vaginal scanner (GE Healthcare, Chicago, IL, USA). The day of ovulation was defined as the day when the ultrasound examination showed that the dominant follicle had disappeared, with a decrease in volume of at least 90% [16].
2) Outcome measures
The main outcome measures for embryo transfer success were the implantation rate, clinical pregnancy rate, miscarriage rate, and multiple pregnancy rate.
4. Controlled ovarian stimulation and oocyte retrieval
All patients underwent controlled ovarian stimulation by a standard long protocol following pituitary downregulation with a gonadotropin-releasing hormone analogue (3.75 mg) and subsequent addition of rFSH (300–375 U/day) until at least three or more follicles had attained a mean diameter of 18 mm. Oocyte retrieval was performed up to 36 hours after the human chorionic gonadotropin (hCG) trigger injection was administered (5,000 IU).
5. Intracytoplasmic sperm injection and embryo culture
Intracytoplasmic sperm injection was performed 4 to 6 hours after retrieval in all patients. The injected oocytes were individually cultured under mineral oil, in 20-µL droplets of Global Total single-step medium (IVFonline, Guelph, ON, Canada) at 37℃ in an atmosphere of 6% CO2 and 5% O2. Fertilization was checked 16 to 18 hours after injection by the presence of two pronuclei.
6. Embryo grading
Excellent-quality cleavage-stage (day 3) embryos were defined as those with 6 to 8 evenly sized cells, no multinucleation, and ≤10% fragmentation. Excellent-quality blastocysts (day 5 embryos) were defined as having an inner cell mass and trophectoderm with many tightly packed cells or several loosely grouped cells.
7. Freezing-thawing procedure
Day 3 and day 5 embryos were vitrified using the Cryotop method [17]. For freezing, Kitazato vitrification media and the Cryotop (Kitazato, Tokyo, Japan) device were used.
Both day 3 and day 5 embryos of the same patient were thawed on the same day using Kitazato thawing media following the standard protocol. After thawing, the embryos were cultured individually in 20-µL droplets of Global Total medium under mineral oil at 37℃ in 5% CO2 in air until embryo transfer.
8. Embryo transfer
All frozen embryo transfers were performed in natural cycles. Two embryos (1 cleavage-stage and 1 blastocyst-stage) were transferred simultaneously to the patients in the MDET group. Two blastocyst-stage embryos were transferred to the patients in the BDET group. In all cases, only excellent-quality (morphologically high-grade) frozen-thawed embryos were used. All embryos were transferred 5 days after ovulation using a Frydman embryo transfer catheter (CCD, Paris, France) previously washed with culture medium.
9. Luteal support and pregnancy
Luteal support was given in the form of micronized vaginal progesterone pessaries in a dose of 2×300 mg daily starting on the day of ovulation and continuing up to 11 weeks of gestation if pregnancy was confirmed.
Serum hCG levels were measured 14 days after embryo transfer. Transvaginal ultrasound was performed 10 days later to detect and confirm intrauterine pregnancy.
Clinical pregnancies were determined by the detection of foetal heart motion in a transvaginal ultrasound examination at 6 to 8 weeks of gestation.
10. Data collection and statistical analysis
Information was recorded regarding patients' baseline characteristics, including age, BMI, duration and cause of infertility, smoking status, basal FSH level, IVF cycle characteristics, and main outcomes.
Statistical data analysis was performed using SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA). Data were reported as mean±standard deviation. Age, BMI, duration of infertility, smoking status, basal FSH, and the number of oocytes were compared between the studied groups of patients using the Student parametric t-test or the Mann-Whitney nonparametric test, depending on the results obtained from the Kolmogorov-Smirnov normality test. The p-values <0.05 were considered to indicate statistical significance. The percentages of subjects who experienced fertilization, implantation, clinical pregnancies, multiple pregnancies, and miscarriages were compared by the chi-square test or the Fisher exact test when the expected frequency was 5 or lower.
Results
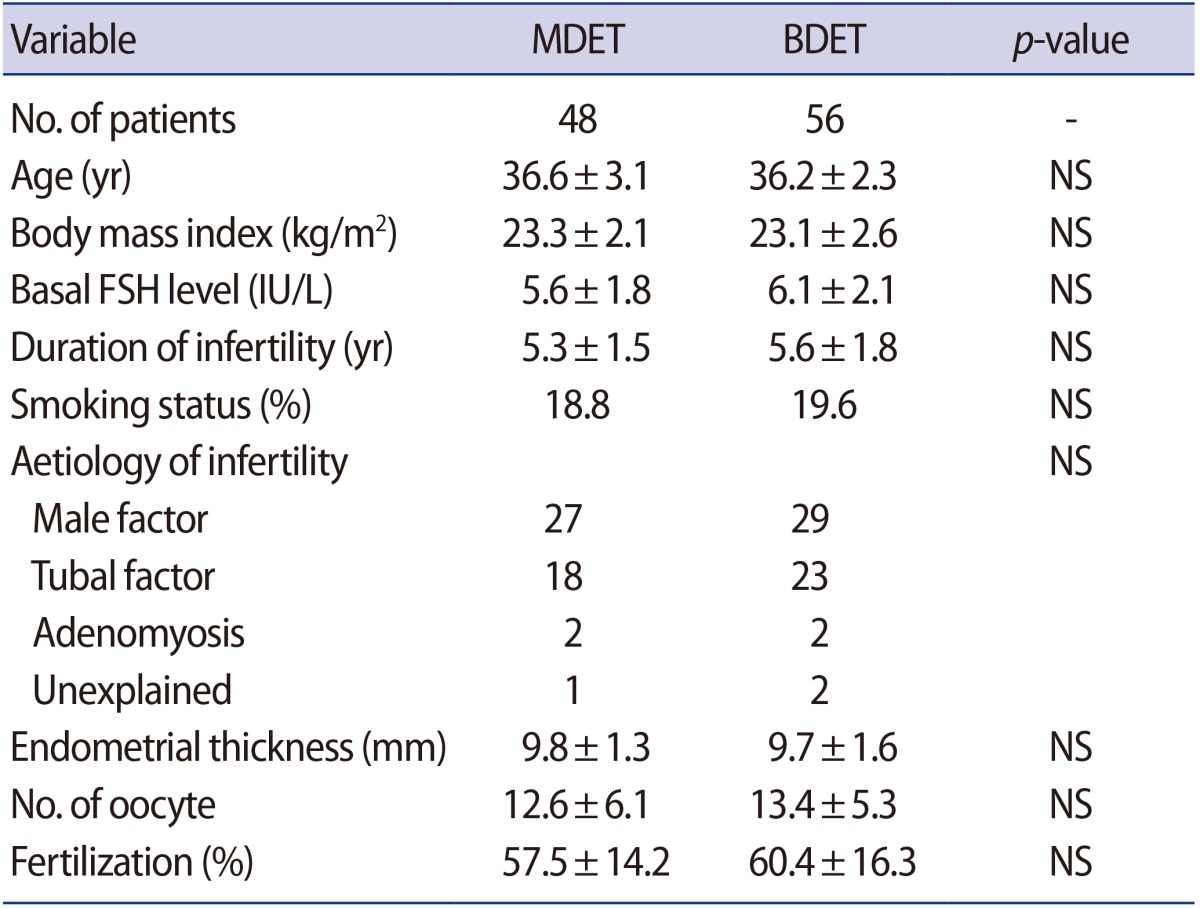
Table 1 compares the main baseline characteristics of the BDET and MDET groups. Both groups were similar in terms of patient age, aetiology and duration of infertility, BMI, basal FSH level, smoking status, endometrial thickness and the mean number of retrieved oocytes. The average fertilization rate was also nearly identical between the two groups (57.5% vs. 60.4%).
The implantation rate was significantly higher in the patients who underwent MDET (60.4% vs. 39.3%) (Table 2). The clinical pregnancy rate was also almost twice as high among those patients (52.1% vs. 30.4%). Moreover, the miscarriage rate in the MDET group was significantly lower (6.9% vs. 10.7%). The multiple pregnancy rate was slightly, but not significantly, higher (27.1% vs. 25.0%) in the MDET group. The observed frequency of monozygotic and dizygotic twins was also very similar between the groups (Table 2).
Discussion
RIF can be attributed to various reasons, and usually is the result of a set of problems that elude easy identification. The main issues involve different aspects of endometrial receptivity and poor embryo quality [1819]. If embryos are of good quality and the culture conditions are optimal, the choice of an appropriate transfer time, the number of embryos, and the developmental stage remain key factors for achieving a successful pregnancy.
The strategies applied by different groups of researchers aiming to find the best conditions and time for embryo transfer include a variety of modifications of standard single and double transfer [2021]. Sequential transfer has been proposed as an alternative method; this technique relies on the variability of the endometrial maturation process, with the goal of increasing the receptivity window [192021222324]. In general, it is assumed that this type of transfer increases the chance of hitting the WOI, since its timing is not constant among all patients in relation to the endometrial response to steroid hormones [1]. However, several authors found no significant improvement in pregnancy rates after applying this technique [2526] and it seems to have some disadvantages compared to standard double-embryo transfer, such as potential catheter-related complications associated with the need of multiple transfer interventions during the same cycle.
Our concept was to take two embryos at different developmental stages and place them at the same time into the uterus to maximize the likelihood of synchronization with the WOI. This alternative ap-proach was applied for the first time in patients with RIF in the current study.
The actual implantation of the embryo into the endometrium occurs 6 to 7 days after fertilization [2728]. The transferred blastocyst-stage (day 5) embryo could be successfully implanted 1 to 2 days later if the endometrium is receptive, while the transferred cleavage-stage (day 3) embryo would have a chance of being implanted 3 to 4 days later (Figure 1). Therefore, MDET should hypothetically guarantee the implantation of at least one embryo during this extended period, with a potential period of implantation of approximately 4 days. The observed 28.6% rate of multiple pregnancies could be explained if these patients had a specific WOI that coincided with the period when both the day 3 and day 5 embryos become ready for implantation (Figure 1).

Blastocyst double-embryo transfer (BDET), multiple double-embryo transfer (MDET), and the implantation window. The increased chance of hitting the window of implantation in MDET is based on the use of embryos at different developmental stages.
When we applied MDET as an alternative to BDET, we observed an almost twofold increase in the clinical pregnancy rate and a significantly reduced risk of miscarriage. It would be expected that the BDET of two day 5 embryos would be successful only when the WOI was not displaced, with implantation occurring 6 to 8 days after ovulation. The hypothesis that the WOI may be displaced by 2 or 3 days in patients with RIF could explain the absence of implantation in a relatively high percentage of the patients undergoing BDET [6]. In our study, the implementation of this innovative approach, which involves the transfer of two embryos at different developmental stages, most likely compensated for the probable shift in the WOI in most cases. Other hypotheses that could possibly explain the obtained results are based on the assumptions that: (1) the transfer of two embryos at different developmental stages ensures the prolonged action of embryonic factors related to the implantation process, such as hCG, soluble human leukocyte antigen G, granulocyte colony-stimulating factor, and preimplantation factor [2930]; or (2) the human endometrium is a heterogeneous structure in which optimal receptivity is achieved at different times in different compartments of the tissue.
In conclusion, in women with RIF, the simultaneous transfer of cleavage- and blastocyst-stage embryos offered a better chance of implantation than BDET. MDET could be considered as a suitable approach for improving IVF outcomes in RIF patients.
Acknowledgments
The authors are thankful to Yoanna Baleva-Jelezarska for proofreading the article.
Notes
This work was supported by Nadezhda Women's Health Hospital, Sofia, Bulgaria.
Conflict of interest: No potential conflict of interest relevant to this article was reported.