Effect of laser-assisted multi-point zona thinning on development and hatching of cleavage embryos in mice
Article information
Abstract
Objective
This study aimed to examine the effect of laser-assisted zona thinning (LAZT) at one or four-points on the blastocyst formation and hatching process in mice with respect to female age.
Methods
Eight-cell or morula embryos collected from superovulated C57BL female mice with different ages (6-11 and 28-31 weeks) were treated with LAZT at one-point (LAZT1) or four-points (LAZT4). The zona pellucida was thinned to more than 70% of its initial thickness by making two holes of 15-20 µm.
Results
In the young mice, LAZT resulted in a significant increase in early hatching and hatching rates compared to the control group (p<0.05). However, in the old mice, LAZT significantly increased blastocyst formation as well as early hatching and hatching compared to the controls (p<0.05). These effects were more remarkable in LAZT4 than in LAZT1 and in aged mice than in young ones.
Conclusion
These results show that multi-point LAZT leads to a significant improvement of blastocyst formation and hatching in mice compared to controls.
Introduction
Hatching is a very important process for successful implantation involving the breaking of the embryo out of the zona pellucida (ZP) at the blastocyst stage. A defect in the hatching stage is considered a possible cause of implantation failure in assisted reproductive technology (ART).
Since the first-ever use of assisted hatching (AH) by Cohen et al. [1], a variety of AH techniques have been used including zona thinning (ZT) and the opening or complete removal of the zona by chemical, mechanical, enzymatic, and/or laser-based methods [23]. Although many studies have reported an increased pregnancy outcome by facilitating the hatching process of embryos [456789], some studies have found no effects or insufficient evidence to determine any effect of AH on live birth rates [1011]. According to a literature review in 2008 by the American Society for Reproductive Medicine, there was no reason to recommend the routine performance of AH in women undergoing ART [12]. Therefore, the effect of AH is still debatable, and an optimum strategy for performing AH remains elusive.
The primary reason for the above mentioned debate is the heterogeneous studies that differ at the following two points: one is the differences in women and/or embryo characteristics such as the age and the endometrial status of women, embryo quality, and asynchronization between the embryos and the endometrium. The other is the differences in the AH methods. Two studies pointed out that the differences in women and/or embryo characteristics were more likely to cause heterogeneity [1314]. However, several studies showed that the effect of AH differed depending on the AH method used [715].
AH is usually performed on day 2 or 3 embryos, or on blastocysts, and the debate on the effect of AH is more common in day 2 or day 3 embryos because the natural hatching process may have polarity and AH at this stage may promote or inhibit the hatching of the blastocysts [16]. However, we do not know the correct hatching site at this stage. Therefore, the choice of the correct AH site might be a very important factor in the improvement of the effect of AH. Nevertheless, few studies on the effects of AH according to the AH site have been reported.
Lasers are now widely used for AH in clinical settings because they are simple, easy, and rapid [17]. Laser AH (LAH) has the following advantages: (1) it saves time; (2) embryos are outside the incubator for less time; and (3) the risk of a temperature increase in the immediate vicinity of the embryos from laser thermal shock is minimized [18]. Therefore, in this study, we examined the effect of laser-assisted zona thinning (LAZT) at one point or multiple points in cleavage-stage embryos on the blastocyst formation and hatching in mice under the hypothesis that multi-point ZT may result in increased chances of hatching.
Methods
This study was approved by the Institutional Review Board of Good Moonhwa Hospital, Korea. All experiments with mice were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institute of Health, approved by the Good Moonhwa Hospital Institutional Animal Care and Use Committee.
1. Animals
For all the experiments, C57BL inbred mice were purchased from the Korea Experimental Animal Center (Daegu, Korea). The mice were maintained on a light-dark cycle, with the light on at 5:00 AM and off at 7:00 PM, and with food and water available ad libitum under a specified pathogen-free condition.
2. Superovulation, zygote collection, and embryo culture in mice
Female mice of two age groups, 6-11 and 28-31 weeks, were superovulated by an intraperitoneal injection with 5 IU pregnant mare's serum gonadotropin, followed by an injection of 5 IU human chorionic gonadotropin (hCG) 48 hours later. Then, the mice were immediately paired with an individual male. Eighteen hours after the hCG injection, female mice with a confirmed vaginal plug were killed by cervical dislocation, and cumulus-enclosed one-cell embryos (zygotes) were retrieved from the oviductal ampulla and denuded by incubation for 1 minute with 0.1% hyaluronidase (Sigma, St. Louis, MO, USA) in Dulbecco's phosphate-buffered saline (dPBS; Gibco BRL, Grand Island, NY, USA). Zygotes were pooled and washed three times in a P1 medium (Irvine Scientific Inc., Santa Ana, CA, USA) with 10% serum substitute supplement (SSS; Irvine).
Only the healthy zygotes were cultured in 30 µL of the P1 medium with 10% SSS for the first 2 days and then, in a blastocyst medium (Irvine) with 10% SSS for the later 2 days in paraffin oil at 37℃ in a 5% CO2 incubator, and the media were changed daily. Blastocyst formation and early hatching rates were determined 96 hours after zygote collection, and the hatching rate was determined 120 hours after zygote collection.
3. Laser-assisted zona thinning
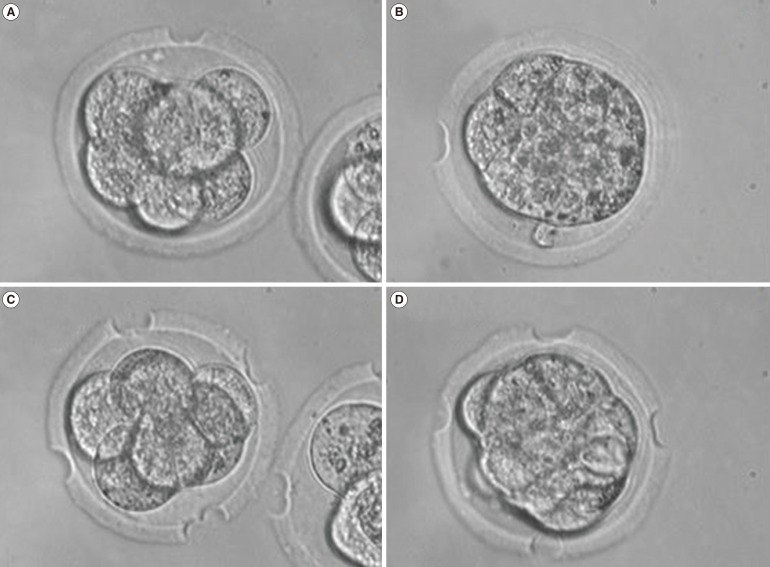
LAH was performed by ZT in 8-cell-stage or morula-stage embryos by using a noncontact 1.5-µm Zilos-tk Laser system (Hamilton Thorne, Beverly, MA, USA). The original culture dish containing the embryos (30-µL drops of the medium) was removed from the incubator and placed on an inverted microscope equipped with a laser system. The point of treatment on the ZP was carefully focused upon and treated with the laser. At this time, the embryos were not stabilized with the holding pipette. One point of treatment on the ZP was randomly chosen, and the sites of four points were determined at 90° intervals from the randomly chosen point. The ZP was thinned to more than 70% of its initial thickness by making two holes of 15-20 µm on the ZP at one point (LAZT1, Figure 1A, B) or four points (LAZT4, Figure 1C, D). All ZT was performed in the empty region without contact to the blastomere in order to minimize the blastomere damage. The laser power was 100%, and the pulse duration was 500 µs. In the control group, embryos were replaced without LAH.
4. Statistical analysis
Statistical analysis was performed using IBM SPSS ver. 19 (IBM Corp. Armonk, NY, USA), and all data were presented as mean±standard deviation. The comparison results of the embryo development and hatching rates were analyzed using the chi-square test and one-way analysis of variance. The value of p<0.05 was considered to be statistically significant.
Results
As shown in Table 1, we performed LAZT in 8-cell embryos of young mice (6-11 weeks). The blastocyst formation rates were 63.8%, 72.6%, and 75.0% in the control, LAZT1, and LAZT4 groups, respectively. No significant difference was found in the blastocyst formation among the three groups. However, the hatching rates at 120 hours after zygote collection were 58.8% and 69.2% in the LAZT1 and LAZT4 groups, respectively. These rates were significantly higher than the rate of 29.3% of the control group (p<0.001). The hatching rate 120 hours after zygote collection in the LAZT4 group was higher than that of the LAZT1 group, but there was no significant difference. The early hatching rate at 96 hours after zygote collection was also significantly higher in the LAZT groups than in the control group (p<0.001).
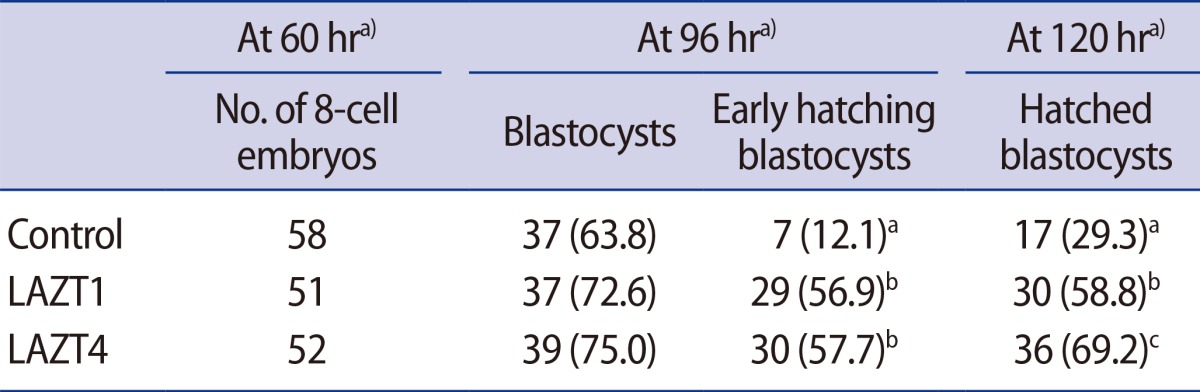
Outcome of blastocyst formation and hatching after LAZT in the 8-cell stage of young mice (age, 6-11 weeks)
LAZT was performed in morula-stage embryos of young mice as in the 8-cell-stage embryos. The blastocyst formation rates in the control, LAZT1, and LAZT4 groups were 67.3%, 77.8%, and 83.3%, respectively, with no significant differences among the three groups. However, the hatching rates 120 hours after zygote collection were significantly higher in the LAZT1 (61.1%) and LAZT4 (77.8%) groups than in the control group (17.3%) (p<0.001). The early hatching rate was also significantly higher in both LAZT groups than in the control group (p<0.001) (Table 2). Figure 2 shows the representative photographs for blastocyst formation and early hatching 24 hours after LAZT in the morula-stage embryos.
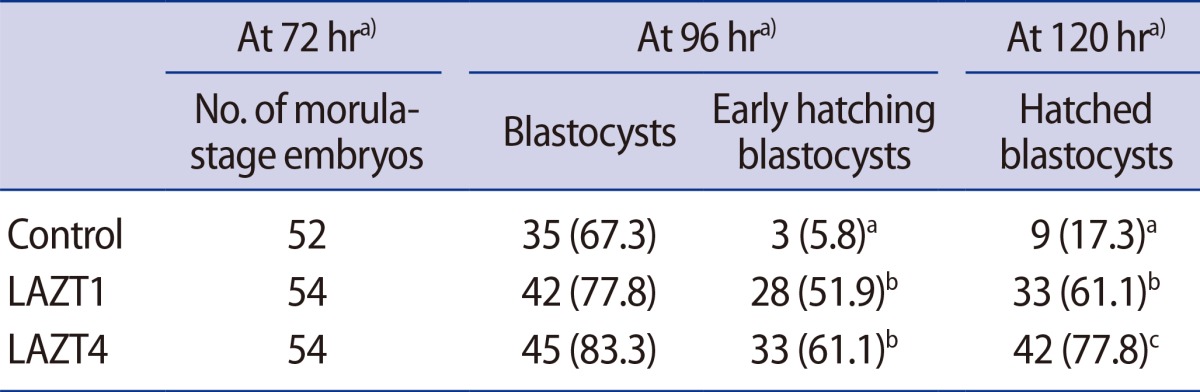
Outcome of blastocyst formation and hatching after LAZT at the morula stage of young mice (age, 6-11 weeks)
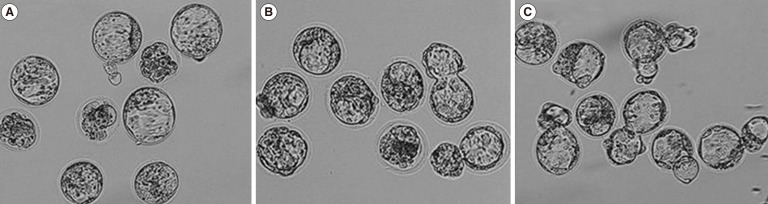
In young mice (6-11 weeks), the representative photographs for the formation and early hatching of blastocysts 24 hours after laser-assisted zona thinning (LAZT) in the morula-stage embryos. (A) Control, (B) LAZT1, (C) LAZT4. ×100.
Next, in order to understand whether the effect of LAZT differs with the female age, LAZT was performed in 8-cell- and morula-stage embryos of aged mice (29-31 weeks). The blastocyst formation rates were 37.3%, 67.3%, and 82.7% in the control, LAZT1, and LAZT4 groups, respectively, with a significant difference between the control group and the LAZT4 group (p=0.005). However, the hatching rates 120 hours after zygote collection were 11.8%, 42.3%, and 65.4% in the control, LAZT1, and LAZT4 groups, respectively, and a significant difference was found between both LAZT groups and the control group (p=0.001). Early hatching rates were also significantly higher in both LAZT groups than in the control group (p=0.007) (Table 3).

Outcome of blastocyst formation and hatching after LAZT at the 8-cell stage of aged mice (age, 28-31 weeks)
Table 4 shows the outcome of embryo development and hatching after LAZT in morula-stage embryos of old mice. The blastocyst formation rates were 40.0%, 82.4%, and 98.0% in the control, LAZT1, and LAZT4 groups, respectively. A significant difference was found between the control and the LAZT groups (p<0.001). The hatching rates 120 hours after zygote collection were significantly higher in the LAZT4 (64.7%) group than in the control group (22.0%) (p=0.008). The early hatching rates were also significantly higher in both LAZT groups than in the control group (p=0.001). Figure 3 shows the representative photographs for the formation and early hatching of blastocysts 24 hours after LAZT at the morula stage of aged mice.

Outcome of blastocyst formation and hatching after LAZT at the morula stage of aged mice (age, 28-31 weeks)
Discussion
This study shows that LAZT significantly improves both the embryo development rate with respect to the blastocysts and the hatching of blastocysts in mice. This effect of LAZT is more prominent in the case of multi-point ZT than in the case of one-point ZT. This is the first study to demonstrate the effect of laser-assisted multipoint ZT on blastocyst formation and hatching in mice.
At least three factors are necessary to be considered for the induction of effective hatching in blastocysts and for ensuring a successful pregnancy outcome. The first is the site of AH. It has been known that a natural hatching site is in close proximity to the inner cell mass (ICM) of blastocysts in humans and at the side opposite to the ICM in mice [1920]. The hatching rate was significantly higher for AH performed near ICM than for AH performed at the side opposite to the ICM in humans [16]. However, in human ART, embryo transfer into the uterus is usually done in embryos that are 2 or 3 days old (that is, 4-cell or 8-cell embryos), and the exact site of hatching at this time is not known. Therefore, the determination of the site of AH is particularly important at these embryo stages. We performed ZT at multiple points in cleavage-stage embryos rather than at one point in order to solve this problem and to increase the possibility of natural hatching polarity. As expected, the hatching rate was remarkably higher in the LAZT4 group than in the LAZT1 group, particularly for aged mice. This result implies that our multi-point ZT is sufficient to improve the chances of hatching compared to one-point ZT.
Second, the determination of the hole size of AH is important to improve the efficacy of AH. To date, there seems to have been a general consensus that larger holes are better for AH [1920]. In fact, some studies have reported that holes larger than a quarter [13], one-third [6], two-thirds, or one-half of the zona diameter result in successful hatching or a relatively high pregnancy rate in humans [2122]. However, a large zona opening or considerable ZT during or after AH may cause damage to the embryos including trapping of more of the larger ICM [212324]. This result suggests that the choice of an appropriate size for AH should be necessarily considered to minimize the risk of direct damage. In this regard, in our present study, we performed ZT to 70%-90% of the initial ZP thickness by making holes of 15-20 µm in the empty region without making contact with the blastomere.
The third is the AH method. This method is designed to easily manipulate the embryos, consume less time, and minimize the damage to the embryos. In this respect, it has been widely recognized that laser AH may fulfill these requirements, and in the present experiment, we used this method.
Another interesting finding of the present study is that laser-assisted ZT stimulated the embryo development rate to blastocysts and, in turn, induced early hatching of blastocysts. This finding is consistent with the result of Miyata et al. [16], who showed that the initiation of hatching occurred earlier in the AH group than in the blastocysts without AH. It is known that electrical stimulation stimulates egg activation. Therefore, it is believed that the early embryo development rate by LAZT may be the effect of electrical stimulation.
Older age in females has been considered an indication for AH, but the effect of AH is still under debate: a study showed a positive effect of AH in patients with advanced age (>38 years) [25], but a recent meta-analysis review reported relatively little effect of AH on clinical pregnancy in aged females [9]. Most studies performed AH in patients younger than 36 years, and some showed a positive effect of AH [61326]. However, one study reported the harmful effects of AH, and another study reported that slightly lower but not statistically different clinical pregnancy rates were observed in the AH group than in the control group among the poor prognosis patients (age>37 years or FSH=10 IU/L) [2728]. One of the main causes of these different outcomes may be the difference in the AH method used. In this respect, this study investigated the effect of AH on female mice with an advanced age of 28-31 weeks; these female mice are comparable to women around 40 years of age. Although this is a mouse model, not a human female model, the effect of LAZT was more remarkable in the older females than in the younger ones. Therefore, a further examination of the effect of our ZT on aged women can contribute to the confirmation of the effect of LAH on aged females. If this effect of AH is clearly confirmed in aged women, it may also be a promising and important new strategy for the improvement of female age-related infertility.
The possibility that multiple shots of a laser beam may pose potential harm to embryos can be raised. It has been demonstrated that embryos at the 8-cell stage respond to a thermal shock by the induction of a heat shock protein [29]. In addition, a study of 134 children born after LAH reported that there was no evidence of LAH leading to an increase in chromosomal abnormalities or congenital malformations [30]. These results suggest that the potential risk of multiple shots of a laser beam can be partly excluded. However, these results do not absolutely preclude the possibility of other underlying abnormalities, and further long-term studies on this topic are needed.
In conclusion, this study showed that multi-point LAZT at 8-cell embryos was more effective in the improvement of the formation and hatching of blastocysts and that this effect was more remarkable in aged mice than in young mice. In turn, these results suggested that this study may contribute to the establishment of an optimal LAH technique that can be effectively applied to human in vitro fertilization and embryo transfer.
Notes
This study was supported by a grant from the Dong-A University research fund.
Conflict of interest: No potential conflict of interest relevant to this article was reported.