The role of methylenetetrahydrofolate reductase C677T polymorphism on the peripheral blood natural killer cell proportion in women with unexplained recurrent miscarriages
Article information
Abstract
Objective
To examine the association between methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism and hyperhomocysteinemia in women with unexplained recurrent miscarriages (RM) and to investigate the association between MTHFR genotype variants and alloimmune activation, proportion of peripheral blood natural killer (pbNK) cells.
Methods
A total of 39 patients with a history of two or more unexplained miscarriages were recruited to this study. The controls were women who had a live birth without a history of RM (n=50). The proportion of pbNK cells was measured by flow cytometry. Plasma homocysteine levels and the incidence of the MTHFR variant of the RM and control groups were compared. The proportion of pbNK cells was compared to the MTHFR variants in the RM group.
Results
No differences were found between the two groups' mean plasma homocysteine levels (7.6±1.5 µmol/L vs. 7.1±2.1 µmol/L) or incidence of the MTHFR genotype variant (CC, 35% vs. 33%; CT, 40% vs. 53%; and TT, 25% vs. 14%). In the RM group, individuals with the TT variant (7.7±1.1 µmol/L) had higher homocysteine levels than those with the CC and CT variants (7.4±1.9 µmol/L and 7.4±1.2 µmol/L) and those with the CT variant (19.2±8.1%) had a higher proportion of CD3-/CD56+ pbNK cells than those with the CC and TT variants (17.7±6.6% and 17.9±7. 0%), but the results of both comparisons were statistically insignificant.
Conclusion
These preliminary results show no difference in plasma homocysteine levels between the RM and control groups or among MTHFR genotype variants in the RM group, which may suggest that the plasma homocysteine level is difficult to use as a predictive marker of RM in the Korean population. A study of a larger number of patients is needed.
Introduction
Recurrent miscarriage (RM) has been a challenging topic in reproductive medicine and is observed in 5% of pregnant women. The causes of RM are identified in only 50% of patients, and the remaining 50% remain unexplained [1]. Unexplained RM involves immunologic, thrombophilic, and environmental factors. In addition to these factors, maternal hyperhomocysteinemia is known as a risk factor for RM [2-4].
Hyperhomocysteinemia is often associated with lowered concentrations of B vitamins, especially of folate. Although insufficient supplementation of folate is one of the reasons for hyperhomocysteinemia, insufficient dietary intake cannot completely explain hyperhomocysteinemia because diets usually do not lack vitamins. The maternal methylenetetrahydrofolate reductase (MTHFR) genotype was found to be major genetic determinant of hyperhomocysteinemia and some studies have reported an association between the MTHFR genotype variant and RM [5-8]. However, the mechanism by which homocysteine causes RM remains elusive. Several hypotheses have been proposed to explain the role of hyperhomocysteinemia in RM. Homocysteine by itself can be embryotoxic [9] or it can potentially interact with hemostatic genetic determinants, thereby increasing the thrombogenic potential [10,11].
Most diseases, such as cardiovascular, autoimmune, and neurodegenerative diseases, are accompanied by hyperhomocysteinemia, which is associated with immune system activation.
In these patients, close associations between hyperhomocysteinemia and the Th1 immune activation, which is reflected by an elevated serum concentration of immune activation marker, neopterin have been reported [12]. Thererfore, a cell-mediated Th1 immune response could be involved in hyperhomocysteinemia associated disease.
However, little is known about the role of homocysteine in the immunologic condition underlying RM of allogenic graft. Identifying the interaction between homocysteine and alloimmunity may allow for a better understanding of the mechanism by which hyperhomocysteinemia causes RM.
The current study was undertaken to examine the association between MTHFR C677T polymorphism and plasma homocysteine concentration in RM and to investigate the association between the MTHFR genotype variant and alloimmnune activation, especially the proportion of peripheral blood natural killer (pbNK) cells, one of the underlying immunologic determinants of RM.
Methods
1. Patients
A total of 39 patients with a history of two or more unexplained pregnancy losses were recruited to this study. Patients were screened for uterine anomaly, parental chromosomal anomalies, anti-phospholipid syndrome (anticardiolipin antibodies and lupus anticoagulant), infection (chlamydia trachomatis, ureaplasma), autoimmunity (anti-thyroglobulin antibodies), and those with positive findings on any of the screening tests were excluded from the study.
A group of 50 fertile couples who had a live birth or were pregnant at 24 gestational weeks over without a history of miscarriages were enrolled as the control group.
The institutional review board of Cheil General Hospital and Women's Healthcare Center, Kwandong University College of Medicine approved this study in August, 2008.
2. Biochemical measurements
Venous blood was collected in EDTA-containing tubes. Plasma was promptly separated by centrifugation at 1,000 rpm for 15 minutes. Plasma homocysteine was determined within 1 hour after blood collection by fluorescent polarizing immunoassay in 49 out of 50 fertile controls. The reference range was 4.5 to 10.6 µmol/L for homocysteine, 1.1 ng/mL to 20 ng/mL for folate and 214 pg/mL to 914 pg/mL for vitamin B12. The intra- and inter-assay coefficients of variation of homocysteine were 1.8% and 2.2%, respectively.
The cut-off value for hyperhomocysteinemia was defined as 11.3 µmol/L, that is, a plasma homocysteine concentration above either the 95th percentile of the distribution of the 49 fertile controls. The mean homocysteine level was 7.2±1.9 µmol/mL (range, 4.2-13.4 µmol/mL) and the 95th percentile level was 11.3 µmol/mL in the fertile control group (Figure 1).
3. MTHFR genotyping
DNA was extracted from leucocytes with a DNA extraction kit (Intron, Seongnam, Korea) according to the manufacturer's protocol. DNA fragments containing the 677th base of MTHFR were amplified with a GeneAmp PCR machine (Perkin Elmer 2400, Foster City, CA, USA), using a sense primer (5'-TGA AGG AGA AGG TGT CTG CGG GA-3') and an antisense primer (5'-AGG ACG GTG CGG TGA GAG TC-3').
4. Flow cytometric analysis of pbNK cells
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized venous blood samples from the study groups using Ficoll-Hypaque (Amersham Biosciences, Piscataway, NJ, USA) density centrifugation and washed twice with phosphate-buffered saline (PBS). 100 µL of PBMCs was washed in PBS with 1% heat-inactivated fetal bovine serum and 0.09% w/v sodium azide (staining buffer) twice, followed by staining with the fluorochrome-conjugated monoclonal antibodies specific for cell surface antigens. Anti-CD16-FITC/CE56-PE (mouse monoclonal) (Beckman Coulter, Fullerton, CA, USA) was used to detect pNK cells. Appropriate isotype controls were used for each antibody. The cell pellets were then fixed and permeabilized for 20 minutes using 250 µL of Cytofix/Cytoperm solution (Pharmingen, San Diego, CA, USA). Immunofluorescence and two-color flow cytometric analysis were performed using a FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA, USA) with a computer interfacing to BD CellQuestPro for full-list-mode data storage, recovery, and analysis. The gate was set on the lymphocyte region by characteristic forward and side scatter parameters. For each sample, 5×106 PBMCs were evaluated and at least 10,000 cells were analyzed. After flow cytometric analysis, 15% of the pNK cell fraction among the lymphocytes was defined as the cut-off level for the immunologic factor underlying RM. The proportion of CD3-/CD56+ pbNK cells was calculated to evaluate the association with the MTHFR genotype and homocysteine concentration.
5. Statistical analysis
Comparisons of homocysteine and vitamin concentrations between individuals were performed using a Student's t-test and chi-square test. One-way analysis of variance was used to assess the differences in continuous variables among the different genotypes. The level of significance was set at a p-value <0.05. All statistical analyses were performed using SPSS ver. 11.5 (SPSS Inc., Chicago IL, USA).
Results
1. Comparison between the RM group and fertile controls
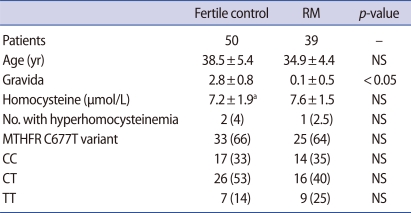
The mean plasma homocysteine levels were comparable between the RM group and fertile controls; the mean homocysteine level of RM was 7.6±1.5 µmol/mL and that of the 49 fertile controls was 7.2±1.9 µmol/mL. There was no difference in the incidence of the MTHFR C677T genotype variant. The number of normal homozygotes (CC) of MTHFR C677T was 14 (35%) in the RM group vs. 17 (33%) in the control group; the heterozygous variant (CT), 16 (40%) vs. 26 (53%), and the homozygous variant (TT), 9 (25%) vs. 7 (14%), respectively, without significant differences. The proportion of individuals with hyperhomocysteinemia in the RM group (2.5%) and fertile controls (4.0%) was comparable (Table 1).
2. MTHFR C677T variant and plasma homocysteine levels in the RM group
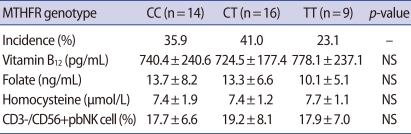
There was a significant negative correlation between folate and homocysteine level (p=0.007), (data was shown). There was no significant difference in the folate or vitamin B12 levels among those with each of the MTHFR genotype variants in the RM group. Individuals with the TT genotype had higher homocysteine levels than those with the CC and CT genotype, but the difference was not significant (Table 2).
3. MTHFR C677T variant and proportion of CD3-/CD56+ pbNK in the RM group
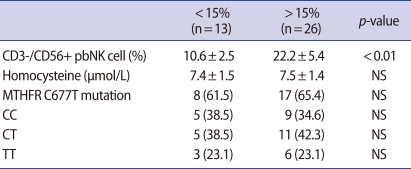
The proportion of CD3-/CD56+ pbNK cells tended to decrease as the plasma homocysteine level increased, but the difference was not significant (Figure 2). Individuals with the CT genotype had a higher proportion of CD3-/CD56+ pbNK cells than those with CC or TT genotype, but again, the difference was insignificant. There was no significant difference in the homocysteine level above or below the CD3-/CD56+ pbNK cell threshold value of 15% (Table 3).
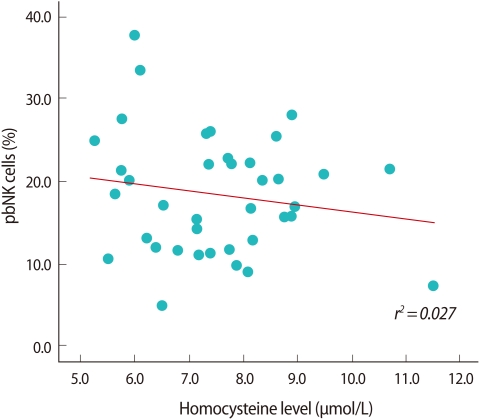
Correlation between plasma homocysteine level and proportion of peripheral blood natural killer cell (pbNK cell).
Discussion
Homocysteine is one of the thiols, a group which includes other endogenous thiols, cysteine, and cysteinyglycine, all of which function as endogenous antioxidants involved in maintaining the pro-oxidant-antioxidant balance in human tissues. Homocysteine is formed from methionine metabolism and can either be remethylated to methionine or transsulfurized to cystathionine. The remethylation reaction depends on 5-methyltetrahydrofolate as the substrate and vitamin B12 as the cofactor, and the transsulfuration reaction depends on the availability of vitamin B6 as a cofactor. Methyl-tetrahydrofolate is formed in a reaction catalyzed by the enzyme MTHFR, the activity of which influences the remethylation of homocysteine to methionine.
Several causes for hyperhomocysteinemia are known. Not only an acquired insufficient supply of the B vitamins folic acid and vitamin B12, but also inherited defects in homocysteine metabolism, mutation C677T in MTHFR play an important role. Mild to moderate hyperhomocysteinemia is associated with detrimental effects on reproductive outcome, ranging from congenital malformations and miscarriages to pregnancy-induced hypertension, low birthweight [2,13], and elevated homocysteine concentrations are observed in patients with cardiovascular [14] and autoimmune as well as neurodegenerative disease [15]. Nelen et al. [4] published a meta-analysis showing a significant association between hyperhomocysteinemia and recurrent early pregnancy loss (odds ratio [OR], 2.7; 95% confidence interval [CI], 1.4-5.2). However, our study failed to show the difference in plasma homocysteine levels between RM and fertile controls, which would be due to periconceptional folic acid supplementation to prevent neural tube defects. This is a limitation of our study because we did not discriminate between women with and without folic acid supplementation because the study was performed retrospectively. Because our study failed to show the difference in plasma homosteine levels between the RM and fertile groups, we cannot firmly conclude that plasma homocysteine level may not be used as predictive marker of RM. A well controlled prospective study is necessary to clarify whether the plasma homosteine level is a causative marker of RM.
The presence of folate and homocysteine in ovarian follicular fluid has previously been demonstrated [16]. Recently, there have been reports of the detrimental effect of a high homocysteine concentration in follicular fluid on embryo quality. Physiological levels of ROS in follicular fluid are necessary for oocyte maturation, ovulation, and fertilization. Excessive scavenging of ROS by thiols, homocysteine has a negative effect on IVF indicating that physiological levels of ROS are essential for normal fertilization [17-19]. Therefore, both low and high concentrations of homocysteine in follicular fluid are associated with reduced embryo quality, suggesting that there must be an optimal level. Although we did not evaluate homocysteine and folic acid levels in follicular fluid, there was significant negative correlation between plasma folate and homocysteine level in RM (p=0.007). We can suggest that the deficiency of plasma folate could have a harmful effect on human reproduction by defective homocysteine metabolism.
Miscarriage remains the most common complication of pregnancy and 2-5% of couples suffer recurrent miscarriage. The cause of miscarriage in 50% of women with RM remains unexplained despite thorough investigations. A number of possible etiologies have been proposed to explain the unexplained RM. Immunological factors are involved in the pathogenesis of RM, and maternal hyperhomocysteinemia is known as a risk factor for recurrent embryo loss [2-4].
Maternal adaptation to the immunological responses, specifically humoral or cellular immunological responses, to the allogenic embryo is necessary for successful pregnancy. The alterations in cellular immunity have been examined and a significant increase in pbNK cells is associated with recurrent miscarriage (RM). A mild to moderate increase of pb CD56+ NK cells (12-18%) in 37.3% of women with RM and a marked increase (>18%) in 14.7% of women with RM was reported [20]. Down-regulation of NK cells in women with RM is associated with a favorable pregnancy outcome [21]. Emmer et al. [22] found that women with RM whose subsequent pregnancy progressed to term delivery had a lower NK cell number than women whose subsequent pregnancies miscarried again. Despite the epidemiologic evidence for a relationship between hyperhomocysteinemia and RM, the role of homocysteine in the pathogenesis of the diseases remains to be demonstrated. One of the pathologic mechanisms is suggestive of immune system, Th1 immune activation based on studies in patients with cardiovascular, autoimmune, and neurodegenerative disease. In case of coronary artery disease, Th1 immune activation marker, neopterin concentrations also correlated well with homocysteine and inversely with folate concentrations (both p<0. 01) [23]. A close association was found between increased homocysteine and neopterin concentrations in patients with Parkinson's disease [15] and similar findings were also found in patients with rheumatoid arthritis [24]. Homocysteine activates the immune system and enhances the inflammatory process. Many authors have demonstrated that homocysteine has an stimulatory effect on Th1 cytokines, IL-6, IL-12, and IL-18, which through activation of intracellular molecules, the nuclear factor kB [25,26]. In rheumatoid arthritis, it has been reported that homocysteine enhances cytokine production in synoviocytes [27]. However, little is known about the effect of homocysteine on immunologic conditions concerning pbNK. Our present study showed a negative correlation between the proportion of CD3-/CD56+ pbNK cells and homocysteine levels, but the result cannot reach a statistically significant power. This is due to the small study population of this preliminary study. Therefore, a large-scale study would clarify the relationship between pbNK cells and homosteine level as an etiologic mechanism of RM which cause inflammatory process. Although our study fails to show a statistically significant correlation between the proportion of pbNK cells and plasma homocysteine level, it is valuable in that it is the first study to evaluate the association between homocysteine level and pbNK cells as one of the underlying immunologic determinants of RM.
Results are still conflicting regarding the association between the MTHFR genotype and RM. Nelen et al. [4] reported on a meta-analysis showing a significant association between the MTHFR TT-genotype and RM (OR, 1.4; 95% CI, 1.0-2.0). However, another meta-analysis showed that the MTHFR TT-genotype was not associated with RM (OR, 1.21; 95% CI, 1.04-1.40) [28]. In our present study, comparing the incidence of the MTHFR genotype variant between the RM group and fertile controls showed no difference and the homocysteine level tended to be higher in the TT genotype, but was not significantly different.
According to our present study, the plasma homocysteine level, hyperhomocysteinemia is difficult to use as a predictive marker of RM. However, our study is limited in that no discrimination was made between women with and without folic acid supplementation because of its retrospective study design. A study of a larger number of patients, prospectively designed and controlled, is necessary to clarify the association between the MTHFR genotype variant, hyperhomosteinemia, folate deficiency, and the proportion of pbNK in patients with unexplained RM.
Notes
No potential conflict of interest relevant to this article was reported.