Beneficial effects of intraovarian injection of platelet-rich plasma in women with poor ovarian response
Article information
Abstract
Objective
Infertility can result from a diminished ovarian reserve, but a potential remedy exists in the form of platelet-rich plasma (PRP) administration. This treatment involves both biological factors and tissue trauma mechanisms, which stimulate folliculogenesis, making it a promising and effective strategy. We assessed the impact of direct PRP injections into the ovaries on the fertility outcomes of women classified as poor responders.
Methods
A quasi-experimental study was conducted from April 2021 to December 2022, focusing on patients classified as POSEIDON grade 3 or 4. PRP injections were administered into both ovaries. After 3 months, data were collected on anti-Müllerian hormone (AMH) level, follicle-stimulating hormone (FSH) level, and the numbers of oocytes, mature oocytes, and good-quality embryos following ovarian stimulation. We then compared the data from before and after PRP injection.
Results
This study included 50 women, with a mean of 39 years (interquartile range [IQR], 35 to 43) and 4 years (IQR, 2 to 6) for age and infertility duration, respectively. FSH levels decreased after treatment, while AMH levels and the numbers of oocytes, metaphase II oocytes, and high-quality embryos increased. However, only the increase in high-quality embryos was significant. The pregnancy and spontaneous pregnancy rates were 20% and 14%, respectively. Notably, women with secondary infertility exhibited a significantly higher pregnancy rate than those with primary infertility.
Conclusion
Ample evidence suggests that PRP can enhance ovarian function. However, further studies are needed to identify the appropriate candidates for this procedure, establish the optimal PRP preparation method, and standardize the procedure for its adjuvant use in assisted reproductive technology cycles.
Introduction
As a woman ages, her likelihood of conceiving naturally diminishes due to the depletion of her ovarian oocyte reserve. Ovarian insufficiency occurs when the number of oocytes drops below a critical level. This decline begins subtly around the age of 32 years and accelerates noticeably after the age of 37 years [1].
As an individual grows older, fertility typically decreases due to a variety of factors [2]. These include an increased probability of various issues that reduce fertility, a heightened risk of spontaneous abortion, and an elevated risk of aneuploidy [3].
Patient-Oriented Strategy Encompassing IndividualizeD Oocyte Number (POSEIDON) is a recently established classification system. It is particularly appropriate for patients with diminished ovarian reserves or those who demonstrate a suboptimal response to exogenous gonadotropins. Four groups have been established in this system based on both qualitative and quantitative parameters, including age, antral follicle count, and anti-Müllerian hormone (AMH) level. Patients in POSEIDON classes 3 or 4 have a low functional ovarian reserve [4].
Ongoing research is dedicated to finding an effective solution for the decline or loss of ovarian reserve. This has resulted in the adoption of several strategies aimed at optimizing ovarian function, including intraovarian platelet-rich plasma (PRP) infusion; stem cell injection to the ovaries; the use of antioxidant supplements; and the application of dehydroepiandrosterone, testosterone supplements, and growth hormones as adjuvants in ovarian stimulation [5,6].
PRP has been employed as an experimental treatment for several years. Initially used for experimental purposes, its clinical application was first seen in other medical fields such as dermatology, orthopedics, and plastic surgery [7]. The concept of utilizing PRP to enhance ovarian function was first proposed in Greece. PRP is obtained by centrifuging peripheral blood to extract platelets [8].
Platelet concentrate is composed of more than 700 proteins, including growth factors, immunomodulators, hormones, and other biologically active proteins. Recent research has indicated that the injection of these factors can stimulate angiogenesis, anabolic processes, cell migration, cell differentiation, and proliferation in targeted tissues [9]. Additionally, PRP has been found to influence mitochondrial activity and reduce oxidative stress [10,11].
Aside from biological factors, it is believed that trauma can also influence PRP by disrupting the Hippo pathway. This pathway includes the oncogenic Yes-associated protein/transcriptional co-activator with PDZ binding motif (YAP/TAZ) system. When activated, this system promotes follicular growth. Mechanical factors play a crucial role in regulating this system. When tensile forces within the cytoplasm increase, the YAP/TAZ system becomes activated. In contrast, a decrease in tensile forces inhibits this system. However, it seems improbable that the insertion of a 17-G needle could cause sufficient damage to the ovary to disrupt the Hippo pathway [12].
The present study aimed to expand on the promising results of previous research involving substantial sample sizes. In it, we explored the effects of injecting PRP into the ovaries of individuals with poor response rates.
Methods
The quasi-experimental study took place in the infertility ward of Shariati Hospital, located in Tehran, Iran, from April 2021 to December 2022. This study employed a before and after design to assess the effects of ovarian PRP injections on patients exhibiting poor ovarian response.
This study included patients who received ovarian PRP within a defined period and were classified as POSEIDON 3 or 4. This classification implies that they had an AMH level below 1.1 ng/mL, fewer than five to seven antral follicles, or a history of cycle cancellation due to insufficient follicular growth or the retrieval of fewer than three oocytes.
Patients who had a partner with male factor infertility or a history of amenorrhea lasting more than 6 months were excluded from the study. In this study, the criteria for selecting patients for intraovarian PRP injections varied based on their previous in vitro fertilization (IVF) history. Patients who had previously undergone an IVF cycle were included to form a comparison group. Conversely, patients who had not previously undergone an IVF cycle were included based on the outcomes of the IVF cycle following intraovarian PRP injection.
We collected data from both hospital records and patients’ medical histories. Approval for the study was granted by the Ethics Committee of Shahid Beheshti University of Medical Sciences (approval number: IR. SBMU. SME. REC.1401.081). The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. Informed consent was obtained from each participant.
Regarding PRP injection, the PRP concentrate was administered during the initial follicular phase, specifically between days 3 and 5 of the menstrual cycle. In all cases, the PRP solution was freshly prepared on the day of the injection. This was done by drawing venous blood from the forearm, typically from the medial vein [13].
A collection kit was utilized according to the manufacturer’s instructions (ROOYA GEN PRP kit [Co. SN: 312569]; Arya Mabna Tashkhis), facilitating the collection of 80 mL of blood. The injection was administered using a 17-G single-lumen needle, under the guidance of transvaginal ultrasound.
The physician injected 4 mL of PRP into each ovarian parenchyma, approaching the ovary at a safe distance from the vascular pedicle to prevent hemorrhagic accidents. A single physician performed all sonographic evaluations using a Mindray sonography machine (Mindray) equipped with a 4 to 9 MHz vaginal probe. During the PRP procedure, patients were under conscious sedation anesthesia.
We examined the levels of AMH and follicle-stimulating hormone (FSH), along with the quantities of oocytes, mature oocytes, and good-quality embryos in IVF cycles before and after 3 months of PRP injection. For patients who had undergone multiple IVF cycles before PRP treatment, the comparison was made between their most recent cycle and their first cycle following 3 months of treatment. However, for patients who had not experienced any IVF cycles prior to treatment, only their first cycle post-treatment was considered.
1. Data analysis
The values were calculated using the mean±standard deviation and the median (interquartile range [IQR]). To compare parametric and non-parametric variables before and after the intervention, we respectively utilized the paired t-test and the Wilcoxon signed-rank test.
To ascertain the correlation between successful pregnancy and various factors, including age, secondary infertility, previous AMH levels, and the duration of infertility, we computed odds ratios (ORs). To mitigate any confounding effects, we calculated adjusted ORs along with their corresponding 95% confidence intervals for variables that exhibited p-values <0.3 in multiple models.
The data were analyzed using SPSS version 24 (IBM Corp.), with two-tailed tests applied at a significance level of p≤0.05. Univariate logistic regression was performed to examine the relationships between age, secondary infertility, prior AMH levels, duration of infertility, and successful pregnancy outcomes. Subsequently, variables with a significance level of <0.3 in the univariate analysis were incorporated into the multiple logistic regression model. ORs, along with 95% confidence intervals, were reported for each variable in the model.
Results
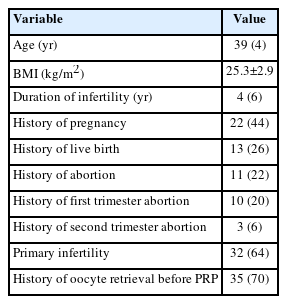
Table 1 presents the basic characteristics and clinical information of women with poor ovarian response undergoing PRP therapy. The study involved 50 women referred to Shariati Hospital. The median of age and infertility duration were 39 years (IQR, 35 to 43) and 4 years (IQR, 2 to 6), respectively. The mean±standard deviation of body mass index were 25.3±2.9 kg/m2. The median duration of infertility was 4 years, with an IQR of 2 to 6 years. Table 1 also provides information about the women’s obstetric history. Our data revealed that 44% of the women had a history of pregnancy, 26% had a history of live births, 64% had primary infertility, and 22% had a history of abortion. Among those with prior abortion, 20% had experienced this in the first trimester and 6% in the second trimester. Furthermore, 70% had undergone oocyte retrieval prior to PRP treatment.
Table 2 presents a comparison of hormone levels and fertility outcomes in women with poor response before and after PRP injection. Prior to PRP injection, the median AMH level was 0.4±0.6 ng/mL (IQR, 0.2 to 0.6), and the median FSH level was 9.1±16.5 mIU/mL (IQR, 8.5 to 17). Following PRP injection, the median AMH level rose to 0.5±0.70 ng/mL (IQR, 0.2 to 1), while the median FSH level decreased to 6±14.09 mIU/mL (IQR, 6 to 15). Statistically significant differences were observed for FSH, but not for AMH (p=0.004 and p=0.48, respectively). The mean number of oocytes retrieved increased from 3.86±2.23 to 5±4.33 after PRP injection, but this difference was not statistically significant (p=0.08). The median number of metaphase II oocytes similarly rose from 2 (IQR, 1.5 to 2.5) to 3.5 (IQR, 3 to 4.5), but the increase was not statistically significant (p=0.08). However, the median number of embryos obtained after PRP injection differed significantly from the number obtained prior to injection (p=0.05). The percentage of patients experiencing abnormal uterine bleeding decreased from 10% to 8% following PRP injection, but this decrease was not statistically significant.
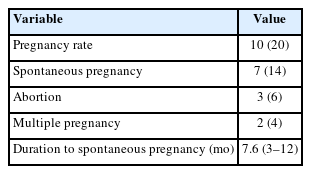
The results revealed that 20% of women with poor response who received PRP injections became pregnant, with 14% experiencing spontaneous pregnancy (Table 3). However, we observed instances of abortion in 6% of cases and multiple pregnancies in 4% of cases. The time frame for achieving spontaneous pregnancy varied between 3 and 12 months, with a median duration of 7.6 months.
After controlling for confounding variables, we found that an increase in age among poor responders decreased the likelihood of successful pregnancy by 17% (Table 4). The data revealed that age was not significantly associated with successful pregnancy in either unadjusted or adjusted analysis (p=0.28 and p=0.13, respectively). Secondary infertility, however, was significantly related to successful pregnancy in both unadjusted (OR, 13.33; p=0.003) and adjusted (OR, 8.57; p=0.023) analyses. In women with secondary infertility, the odds of successful pregnancy were 8.57 times greater than among those with primary infertility.
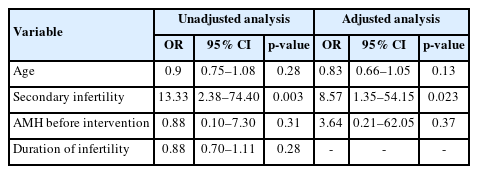
Association between clinical factors and successful pregnancy (unadjusted and adjusted analysis) in poor-responder women
The pre-intervention AMH level did not show a significant association with successful pregnancy in either unadjusted or adjusted analyses. However, an increase in the AMH level prior to the intervention did correlate with a 3.64-fold increase in the likelihood of successful pregnancy. The duration of infertility did not significantly correlate with successful pregnancy in the unadjusted analysis (OR, 0.88; p=0.28).
Discussion
The existence of oogonial stem cells presents a new possibility for treating age-related fertility decline and pathological conditions such as premature ovarian failure. Ovarian rejuvenation may be achieved with this approach. A recent study investigated the potential effects of intraovarian injections of autologous PRP on ovarian rejuvenation [14]. The study involved 50 patients, aged 27 to 40 years, who were anticipated to have a poor response to ovarian stimulation and were categorized as POSEIDON 3 or 4. Additionally, 64% of these patients had a history of ovarian retrieval.
Several reports have demonstrated that women with diminished ovarian reserve or premature ovarian failure experience improvements in follicular count, hormone levels, and successful pregnancy outcomes after undergoing intraovarian PRP treatment [15,16]. Our research corroborates these findings, indicating that PRP positively impacts ovarian function. This is evidenced by increased levels of AMH, decreased levels of FSH, and increases in the numbers of both oocytes and mature oocytes.
The most noteworthy result is the improvement in oocyte quality, which led to the development of high-quality embryos, regardless of any alterations in the ovarian reserve or oocyte count. This topic has attracted considerable attention. In a study conducted by Merhi and Mouanness [17], the application of PRP to the ovaries of infertile women who had previously experienced failed IVF cycles yielded relatively higher embryo euploidy rates. The localized paracrine effect of growth factors present in PRP could potentially improve meiotic aberrations in human oocytes, thereby enhancing euploidy rates [17]. Several in vitro and experimental studies have demonstrated the beneficial effects of PRP. Hosseini et al. [18] conducted research to examine the impact of PRP on the growth and viability of both fresh and vitrified-thawed ovarian follicles.
Several case series and studies have documented pregnancies in women diagnosed with premature ovarian insufficiency (POI) after receiving ovarian PRP injections, either through IVF or spontaneously [13,19]. Consistent with these findings, our study revealed that of the 10 pregnancies observed, seven (70%) occurred spontaneously, while three (30%) were the result of IVF. It is widely accepted that women with POI have a 5% to 10% chance of conceiving naturally without any fertility intervention [20]. However, our research indicates a higher probability, agreeing with the findings of other studies. Interestingly, we found that the pregnancy success rate was significantly higher in individuals experiencing secondary infertility. Our study did not specifically distinguish between primary and secondary infertility in the analysis. Nevertheless, the finding that secondary infertility was associated with a significantly higher OR for successful pregnancy indicates that additional factors may contribute to lower success rates in patients with primary infertility. This suggests that primary infertility, defined as the inability to conceive after 1 year of regular unprotected intercourse in couples without previous live births, may have unique characteristics that our study did not fully reveal.
Aflatoonian et al. [21] reported a satisfactory pregnancy rate of 47% among women who had previously exhibited a poor response, with 50% of these pregnancies resulting in the birth of a live baby. Notably, all pregnancies occurred naturally after PRP administration [21].
Our study population was characterized by its heterogeneity due to the inclusion of patients with diverse IVF backgrounds and treatment histories. We particularly acknowledge a subgroup of patients who had never previously undergone oocyte retrieval, representing a unique subset within our cohort. The observed success rate of 30% observed in this subgroup underscores the potential impact of previous treatment experience on outcomes.
In our study, all clinical and spontaneous pregnancies occurred within 1 year in patients who had undergone ovarian stimulation cycles within 90 days of receiving a PRP injection. Typically, under physiological conditions, the progression of primary follicles to pre-antral follicles takes approximately 120 days [22]. Furthermore, the initiation of the transition from primary to pre-antral follicles could explain the delayed effect of PRP, which was observed 2 to 3 months post-injection, even though the infused cytokines had already broken down. However, many aspects of the paracrine control of folliculogenesis and the contents of PRP remain unclear [9,23].
Although PRP has shown promising results in many cases, a universally accepted method for its preparation remains elusive, as do clear guidelines for identifying suitable candidates for PRP therapy. This absence of standardization could account for the diverse results seen across studies as well as the differing mechanisms of PRP’s effects.
Several methods exist for the processing of whole blood to create PRP, which may involve variations in centrifugation speed and duration, separation techniques (like mechanical or manual pipettes), and the application of activators after preparation. These techniques differ across studies.
Various injection techniques also exist, such as the cortex, medulla, and intraperitoneal methods. Injections can be administered through single or multiple sites, and they can be carried out using either laparoscopic or vaginal routes [9].
Our patients were categorized as poor responders; however, their mean FSH level was 9 mIU/mL. Only three patients had an FSH level exceeding 25 mIU/mL, and none surpassed 40 mIU/mL. Furthermore, only 10% of patients experienced irregular menstruation due to the perimenopausal period. In one previous study, Barad et al. [24] examined 80 consecutive patients with poor ovarian reserve, aged between 28 and 54 years. Poor ovarian reserve was defined as an AMH level below 1.1 ng/mL, an FSH level above 12 mIU/mL, or at least one previous IVF cycle yielding three oocytes within a year.
The study followed women for 1 year following an intraovarian PRP procedure, which entailed the injection of 1.5 mL of PRP into the ovarian cortex, averaging 12 injections per ovary. Despite this, the study findings suggested no statistically significant advantages associated with the intraovarian PRP treatment. Among all the patients, only two (4.7%) experienced ongoing pregnancies [24].
In our analysis, we included cases with identified causes of infertility, such as male factor infertility, tubal factor infertility, and uterine abnormalities. Notably, these factors can influence both the number of embryos and pregnancy rates. While we did not specifically stratify the results based on individual causes of infertility, we maintain that incorporating these cases enhances the overall heterogeneity of our study population. This approach offers a relatively stronger representation of real-world clinical scenarios.
When interpreting the results of our study, it is crucial to recognize the statistical limitations that arise from the relatively small sample size. The inclusion of a limited number of participants could potentially impact the statistical power and generalizability of the findings.
To date, no serious adverse effects associated with ovarian PRP injections, such as vascular injury, organ perforation, infection, abscess formation, or oocyte tissue necrosis, have been reported. Similarly, no adverse effects were observed in the present cohort. Nevertheless, it is important to acknowledge that the long-term effects of this procedure remain unknown. Additionally, a theoretical risk is associated with administering highly concentrated growth factors to tissues, which could potentially induce malignant transformation [9].
In conclusion, for those who have previously been pregnant and are now experiencing infertility primarily due to age-related fertility decline, PRP presents a viable approach to enhance ovarian function and improve response to ovarian stimulation. This could potentially yield successful pregnancy outcomes.
The most notable impact of PRP appears to be on the quality of oocytes and the subsequent embryos. This effect is believed to stem from alterations in the ovarian microenvironment, which enhance angiogenesis and mitigate oxidative and inflammatory effects. Numerous studies have also demonstrated that PRP can stimulate folliculogenesis, resulting in an increased number of follicles and an enhancement in ovarian reserve.
Overall, referring to PRP administration as “ovarian rejuvenation” may not be fully accurate, as any observed benefits might be only temporary. Although this method has not yet been recognized as a standard treatment option for poor responders, the current research indicates that it could be a viable alternative. This is supported by the promising results observed in other studies, particularly in a statistically significant number of poor-responder patients who underwent ovarian PRP treatment.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: AN, SNK. Data curation: AA, MA. Formal analysis: AA, MA. Funding acquisition: MA, SH. Methodology: AN, MA, SNK. Project administration: AN, SNK. Visualization: AN, SNK. Writing-original draft: AA, SH. Writing-review & editing: MA, SH.