Commentary on the new 2022 European Society of Human Reproduction and Embryology (ESHRE) endometriosis guidelines
Article information
Abstract
Endometriosis is a prevalent benign illness defined by the presence of endometrial glands and stroma outside of the uterine cavity, primarily on the ovary, pelvic peritoneum, and rectovaginal septum, resulting in a variety of symptoms, including dysmenorrhea and infertility. Traditionally, prolonged medical therapy has been needed in most cases since a conservative approach to surgery has usually been taken, especially in young women. In 2022, new European Society of Human Reproduction and Embryology (ESHRE) guidelines were published that present different directions for diagnosis and treatment from the past. Furthermore, the guidelines for the diagnosis and management of endometriosis are more precise and applicable than in previous editions. Thus, referring to the representative changes in the new guidelines and important updates will be beneficial for the diagnosis and management of endometriosis. This paper provides a brief overview of these developments.
Introduction
Endometriosis is a prevalent benign illness defined by the presence of endometrial glands and stroma outside of the uterine cavity, primarily on the ovary, pelvic peritoneum, and rectovaginal septum [1]. It is linked to infertility and a variety of problems, including chronic pelvic pain, dysmenorrhea, deep dyspareunia, dysuria, dyschezia, and fatigue [2,3]. However, symptom severity is not usually proportional to the endometriosis stage, and some women with endometriosis may be asymptomatic.
Traditionally, endometriosis has been diagnosed through diagnostic laparoscopy or the histological identification of lesions. However, with recent developments in imaging modalities, the necessity for diagnostic laparoscopy in cases where endometriosis is relatively obvious has been questioned, and concerns have been raised regarding delays in the endometriosis diagnosis due to the diagnostic laparoscopic and histological confirmation criteria.
In 2022, the new European Society of Human Reproduction and Embryology (ESHRE) guidelines were published [4]. Although the new guidelines are not perfect, they provide clearer guidance on many difficult issues, such as medication selection among combined oral contraceptives (COCs), progestogen, and gonadotropin-releasing hormone (GnRH) agonists, and surgical indications for endometrioma in patients preparing for pregnancy.
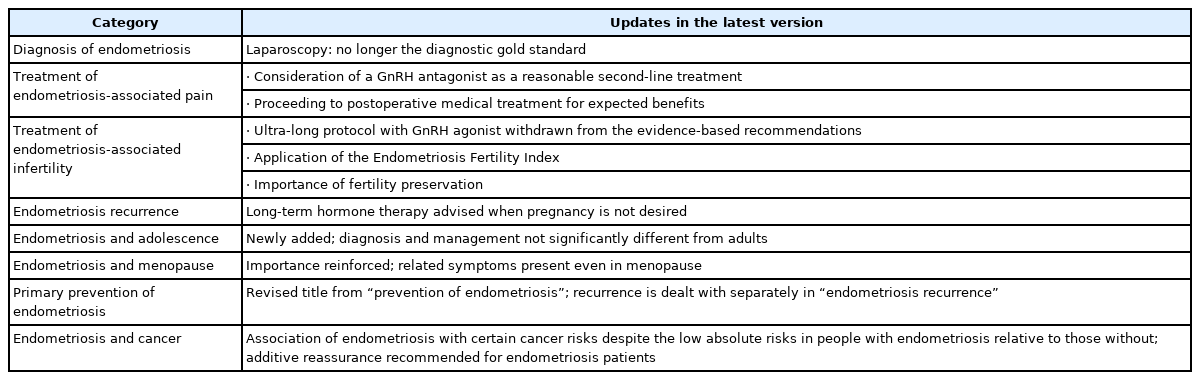
The ESHRE guidelines are one of the most frequently cited endometriosis-related guidelines. It is believed that referring to representative changes in the new guidelines and major updates will be beneficial for diagnosing and treating endometriosis (Table 1). This paper provides a brief overview of these developments.
Diagnosis of endometriosis
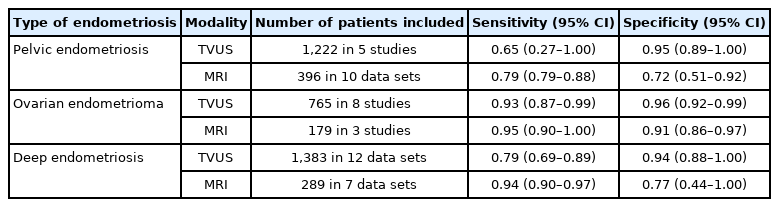
The previous ESHRE guidelines suggested that laparoscopic histologic confirmation of endometriosis was the gold standard for an endometriosis diagnosis. In contrast, according to the new ESHRE guidelines, laparoscopy is no longer the diagnostic gold standard and is now only advised for patients with negative imaging results and/or when empirical treatment is ineffective or unsuitable. This change was based on a meta-analysis regarding endometriosis diagnostic tools. According to a Cochrane review, imaging modalities such as transvaginal ultrasonography and magnetic resonance imaging showed sensitivity and specificity for diagnosing endometrioma and deep endometriosis comparable to a surgical diagnosis (Table 2) [5]. As an added explanation in the new guidelines, the difficulties and limitations of making a noninvasive diagnosis of a superficial disease are described. Despite these limitations, stating that diagnostic laparoscopy is not the gold standard emphasizes the usefulness of imaging modalities for diagnosing endometrioma and deep endometriosis.
Treatment for endometriosis-associated pain
Discomfort related to endometriosis includes dysmenorrhea, dyspareunia, dysuria, dyschezia, and non-menstrual pelvic pain. In prior Korean and international guidelines, the hormonal medical treatment for endometriosis-associated pain has consisted of COCs, progestogens, anti-progestogens, aromatase inhibitors, and danazol. However, in the new guidelines, GnRH antagonists were introduced as a second treatment option, while anti-progestogens and danazol were withdrawn.
1. Introduction of GnRH antagonists as a second-line treatment option
The use of GnRH antagonists to alleviate endometriosis-associated pain can be considered. However, limited data exist on application of GnRH antagonist. Due to their side-effect profile, they are provided as a second-line option (for instance, if hormonal contraceptives or progestogens are ineffective). A study on two identical multi-center double-blind, randomized, placebo-controlled, phase 3 trials of 6-month treatments with oral elagolix at two doses in women with moderate or severe endometriosis-associated pain provided data on treatment efficacy. The proportion of women who met the clinical response criteria for dysmenorrhea and non-menstrual pelvic pain was significantly higher among women who received each elagolix dose (46% in the lower-dose group, 75.8% in the higher-dose group) than among those who received placebo (19.6%). The reductions in dysmenorrhea and non-menstrual pelvic discomfort were noticeable after 1 month and persisted for 6 months. The most frequently reported adverse reactions were hot flushes, headaches, and nausea [6].
2. Changes in other medications
A levonorgestrel-releasing intrauterine system (LNG-IUS) has long been recognized as an effective treatment for endometriosis-associated discomfort. A recent randomized controlled trial (RCT) assigned 103 women with endometriosis-related chronic pelvic pain and/or dysmenorrhea to receive either an etonogestrel (ENG)-releasing subdermal implant or a 52-mg LNG-IUS [7]. Both the ENG implant and the LNG-IUS significantly reduced endometriosis-related pain, dysmenorrhea, and chronic pelvic pain. To alleviate endometriosis-related discomfort, it is recommended that women receive either an ENG implant or an LNG-IUS.
In addition, the Guideline Development Group (GDG) suggested using GnRH agonists as second-line treatments due to their side effect profile. Danazol and anti-progestogens, laparoscopic uterosacral neck ablation, presacral neurectomy, and anti-adhesion medications are no longer included in the recommendations of guidelines because of their harmful effects or lack of extra benefit.
3. Effectiveness of postoperative medical treatment
According to previous guidelines, practitioners should not provide adjunctive hormonal treatment for endometriosis-associated pain following surgery because it does not improve the outcomes of surgery to relieve pain. However, a recent Cochrane analysis by Chen et al. suggested that postsurgical medical therapy might reduce pain recurrence and illness recurrence within 12 months [8]. To improve the immediate success of surgery for pain in women with endometriosis who do not desire pregnancy, postoperative hormonal therapy may be administered.
Treatment of endometriosis-associated infertility
1. Introduction of the Endometriosis Fertility Index
The GDG recommends that the decision to perform surgery should be made in consideration of some factors such as the presence or absence of pain symptoms, patient age and preferences, history of previous surgery, presence of other infertility factors, ovarian reserve, and the estimated Endometriosis Fertility Index (EFI). The EFI was added to the new guidelines for the first time. The EFI staging system predicts non-in vitro fertilization pregnancy rates following surgical endometriosis staging and treatment [9]. The EFI is determined by historical variables (age, number of years of infertility, and prior pregnancy) and surgical variables (the American Society for Reproductive Medicine [ASRM] overall score, the ASRM endometriosis score, and the least function score).
2. No benefit of ultra-long GnRH agonist prior to assisted reproductive technology
The extended administration of a GnRH agonist prior to assisted reproductive technology treatment to increase the live birth rate in infertile women with endometriosis (ultra-long protocol) is no longer suggested due to the lack of evidence supporting its benefits.
The previous recommendation was based on an older Cochrane review regarding GnRH agonist pre-treatment, which included 228 patients from three studies and showed an increased likelihood of clinical pregnancy by more than 4-fold [10]. However, an updated Cochrane review that included eight parallel-design RCTs with a total of 640 participants, concluded that the effect of GnRH agonist pre-treatment (for at least 3 months) was very uncertain, both on the live birth rate as the primary outcome and on secondary outcomes (clinical pregnancy rate, multiple pregnancy rate, miscarriage rate, the mean number of oocytes, and the mean number of embryos) [11]. Another meta-analysis of studies comparing different GnRH agonist protocols (short, long, and ultra-long) also found that different down-regulation protocols did not significantly improve clinical outcomes (implantation rate, fertilization rate, and clinical pregnancy rate) by analyzing RCTs and observational studies (n=21) [12].
3. Importance of fertility preservation
Prior ESHRE guidelines on fertility preservation considered benign disorders to be an indication for fertility preservation, but did not address whether endometriosis was a reason for fertility preservation in particular [13]. A recent large retrospective study by Cobo et al. [14] evaluated the outcome of fertility preservation utilizing vitrified oocytes in 485 patients with endometriomas of at least 1 cm and an antral follicular count of at least 3, finding oocyte survival rates of 83.2% after warming and a cumulative live birth rate of 46.4%. They concluded that fertility preservation was a valid treatment option in patients with endometriosis. Although several issues such as cost-effectiveness and specific indications remain unsolved, practitioners should explore the advantages and disadvantages of fertility preservation with endometriosis patients who have severe ovarian endometriosis.
4. Impact of endometriosis on pregnancy and pregnancy outcomes
Sparse data with low and moderate quality indicated that the behavior of endometriotic lesions during pregnancy was diverse, ranging from complete elimination to increasing growth [15]. Patients should not be encouraged to become pregnant with the primary aim of treating endometriosis because pregnancy does not necessarily result in symptom improvements or slow disease progression. The decidualization of an endometrioma during pregnancy may resemble malignant ovarian tumors in some circumstances, providing a diagnostic conundrum. However, the incidence of this event is unknown (0%–12% prevalence, 17 studies reporting 60 cases) [16]. Endometrioma can manifest differently throughout pregnancy. If an ultrasound examination during pregnancy reveals atypical endometrioma, it is recommended that the patient be referred to a center with the necessary expertise.
Complications directly connected to pre-existing endometriosis lesions are uncommon and likely underreported. These issues may result from decidualization, adhesion formation/stretching, and endometriosis-related chronic inflammation [16]. Although uncommon, these may pose life-threatening conditions that necessitate surgical treatment. Clinicians should be aware that women with endometriosis may have a higher risk of miscarriages and ectopic pregnancies during the first trimester. They should also be informed of uncommon endometriosis-related problems during pregnancy, which include gestational diabetes, preterm birth, premature rupture of membranes, placenta previa, hypertensive disorders and pre-eclampsia, stillbirth, cesarean section, obstetric hemorrhage (placental abruption, antepartum and postpartum bleeding), small for gestational age, admission to the neonatal intensive care unit, and neonatal death. However, since these results are based on low- or moderate-quality research, they should be regarded with caution, and additional antenatal monitoring is not recommended.
Endometriosis recurrence
The recurrence rate of endometriosis has been reported to range from 0% to 89.6% [17]. This variation could be related to differences in the definitions of recurrence, length of follow-up, study design, sample size, type and stage of disease, type of operation, and postoperative medical care [17]. As the high recurrence rate and its significance have been consistently emphasized, they are described in a separate chapter, unlike in the earlier guidelines. For preventing recurrence, ovarian cystectomy instead of drainage/electrocoagulation and postoperative hormonal treatment for at least 18 to 24 months are recommended. The recommended duration of hormonal treatment is based on RCTs. However, in patients who are not immediately seeking conception, long-term hormonal therapy is recommended.
Endometriosis and adolescence
There are scarce data on endometriosis and adolescence. Although there are no major differences between the diagnosis and treatment of endometriosis in adolescents and adults, clinicians treating adolescents should be vigilant regarding several factors. Not only is dysmenorrhea a major symptom of endometriosis, but it is also a highly prevalent occurrence in adolescents, and it takes significantly longer to reach a diagnosis of endometriosis in adolescents than in adults. When previous treatments have failed, clinicians may consider prescribing a GnRH agonist for up to 1 year. If GnRH agonist treatment is considered for young women and adolescents, it should be delivered only after careful evaluation and a discussion of potential adverse effects and long-term health problems with a practitioner in a secondary or tertiary care setting, as recommended by the GDG. As with adults, no studies have addressed the efficacy or utility of fertility preservation—specifically, oocyte cryopreservation—in adolescents with endometriosis. Although the true benefits, safety, and indications for adolescents with endometriosis remain unknown, the GDG recommends informing adolescents about fertility preservation options.
Endometriosis and menopause
The amount of data available regarding the prevalence of endometriosis after menopause is extremely limited. A recent retrospective cohort study described a 4% prevalence of postmenopausal endometriosis [18]. It is hypothesized that menopausal hormone therapy can increase the formation of endometriosis [19] and a variety of factors, some of which are unknown, can also affect the growth of endometriosis. Therefore, it is important for clinicians to be aware that endometriosis might continue to be active and cause symptoms after menopause.
For postmenopausal women presenting with signs of endometriosis and/or discomfort, clinicians might consider surgical treatment as a means of enabling histological confirmation of the diagnosis of endometriosis. The GDG suggests that practitioners emphasize the ambiguity regarding the risk of cancer in postmenopausal women. If a mass is found in the pelvis, a diagnostic workup and treatment should be carried out in accordance with the national oncology standards. Clinicians may consider aromatase inhibitors as a potential therapeutic option for endometriosis-related pain in postmenopausal women, particularly if surgery is not a viable option.
Clinicians should be aware that women with endometriosis who undergo early bilateral salpingo-oophorectomy as part of their therapy have a higher risk of decreased bone density, dementia, and cardiovascular disease. It is also essential to emphasize that women with endometriosis have a higher risk of cardiovascular disease, regardless of whether they have undergone early surgical menopause.
For the management of postmenopausal symptoms in women with a history of endometriosis, combined menopausal hormone therapy may be considered; however, tibolone is no longer recommended as a medical treatment for menopausal symptoms in women with a history of endometriosis.
Extra-pelvic endometriosis
Clinicians should be aware of the symptoms of extra-pelvic endometriosis, which include cyclical shoulder discomfort, cyclical spontaneous pneumothorax, cyclical cough, and enlargement of nodules during menstruation. The diagnosis and treatment of extra-pelvic endometriosis should be discussed by a multidisciplinary team at an institution with sufficient expertise. When possible, surgical excision is the best treatment for alleviating symptoms in women with abdominal extra-pelvic endometriosis. If surgery is inapplicable or unacceptable, hormonal therapy may also be an alternative.
Asymptomatic endometriosis
Clinicians should not regularly perform surgical excision/ablation for asymptomatic endometriosis discovered incidentally during surgery. Practitioners should not suggest medical therapy to women with an incidental endometriosis diagnosis, but routine ultrasound surveillance must be recommended. Even in the absence of convincing data on the benefits of monitoring asymptomatic endometriosis, the GDG recommends considering ultrasound surveillance due to its low cost and safety.
Primary prevention of endometriosis
The objective of primary prevention is to prevent healthy and asymptomatic women from developing endometriosis. Although there is no direct proof of the efficacy of a healthy lifestyle and diet in preventing endometriosis, women can be counseled to adopt a healthy lifestyle and diet, including reduced alcohol use and regular physical activity. The efficacy of hormonal contraceptives for the primary prevention of endometriosis is uncertain. Genetic testing of women with suspected or proven endometriosis should only be undertaken in a research context, citing the newly added text.
Endometriosis and cancer
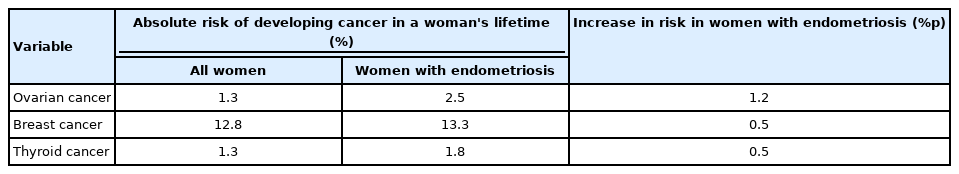
According to the 2014 recommendations, ovarian cancer and non-Hodgkin lymphoma were slightly prevalent among endometriosis patients. Nevertheless, according to a recent systematic review and meta-analysis of 49 cohort or case-control studies, endometriosis was related to a very slight and not statistically significant higher risk of cancer overall (summary relative risk [SRR], 1.07; 95% confidence interval [CI], 0.96–1.16) [20]. Endometriosis was related to an increased risk of ovarian cancer (SRR, 1.93), especially clear-cell (SRR, 3.44) and endometrioid (SRR, 2.33) histotypes, breast cancer (SRR, 1.04) and thyroid cancer (SRR, 1.39) [20]. Although endometriosis is associated with an elevated risk of certain cancers, given the low absolute risks of ovarian, breast, and thyroid cancer in people with endometriosis relative to those without (increases of 1.2%p, 0.5%p, and 0.5%p, respectively) and the uncertainty regarding the risk of other cancers, endometriosis patients can be reassured that their cancer risk is low and comparable to that of people without the disease. Clinicians should educate women with endometriosis who request information on their cancer risk that endometriosis is not associated with an increased risk of cancer. Although endometriosis is related to a somewhat higher risk of ovarian, breast, and thyroid malignancies, the absolute increase in risk relative to the general female population is minimal (Table 3).
Conclusion
The new ESHRE recommendations are clearer and more practical than their predecessors and other guidelines. However, as stated on the opening page of the ESHRE guidelines, clinical practice guidelines do not supersede the clinical decisions made by a healthcare professional for diagnosis and treatment. Ultimately, healthcare professionals must make their own clinical decisions case by case, with consideration of various circumstances.
Notes
Conflict of interest
Jong Kil Joo is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author contributions
Writing–original draft: all authors. Writing–review and editing: JKJ.