Sperm DNA fragmentation negatively influences the cumulative live birth rate in the intracytoplasmic sperm injection cycles of couples with unexplained infertility
Article information
Abstract
Objective
This study aimed to determine the effect of sperm DNA fragmentation (SDF) on the cumulative live birth rate (CLBR) in intracytoplasmic sperm injection (ICSI) cycles in couples with unexplained infertility.
Methods
We conducted a prospective study of 145 couples who underwent ICSI cycles for unexplained infertility. Based on the SDF rate, patients were categorized into a low SDF group (SDF ≤30%, n=97) and a high SDF group (SDF >30%, n=48). SDF was assessed using the acridine orange test on density gradient centrifugation prepared samples. The CLBR was calculated as the first live birth event per woman per egg collection over 2 years.
Results
The high SDF group (SDF >30%) showed a significantly lower CLBR (p<0.05) and a significantly higher miscarriage rate (p<0.05) than the low SDF group (SDF ≤30%). No significant difference was observed in the implantation and cumulative pregnancy rates between the two SDF groups. The total number of embryo transfers was stratified further into fresh and frozen embryo transfers. In the fresh embryo transfers, there were significant differences in the implantation rates, clinical pregnancy rates, and live birth rates (p<0.05) between the low SDF and high SDF groups. However, in the frozen embryo transfers, there were no significant differences in clinical outcomes between the two groups. In the multivariable logistic regression analysis, SDF was a predictor of CLBR (p<0.05) when adjusted for possible confounding factors.
Conclusion
High SDF was associated with a lower CLBR and a higher miscarriage rate in the ICSI cycles of couples with unexplained infertility.
Introduction
Infertility affects approximately 15% of couples of reproductive age [1]. Unexplained infertility refers to couples who fail to conceive despite having a female partner with healthy ovulatory function and patent fallopian tubes and a male partner with standard semen analysis results [2]. Although the underlying reasons for unexplained infertility have not been fully identified, increasing evidence suggests that sperm DNA fragmentation (SDF) should be considered [3-5].
Approximately 25%–80% of couples with unexplained infertility have elevated SDF values [4-6]. Reduced pregnancy and live birth rates with increased miscarriage rates were observed in couples with idiopathic infertility and >25% SDF after in vitro fertilization (IVF) cycles [4]. Non-male factor infertility couples with SDF ≥30% have shown lower rates of normal cleavage speed, high-quality embryos at day 3, blastocyst development, blastocyst quality, and implantation in intracytoplasmic sperm injection (ICSI) cycles [7].
Previously, intrauterine insemination (IUI) and IVF were the first-line treatments for couples with unexplained infertility [5]. A meta-analysis concluded that, in cases of well-defined unexplained infertility, the use of ICSI was favored over IVF to increase fertilization rates and reduce the risk of total fertilization failure (TFF) [8]. Approximately 5%–25% of IVF cycles lead to TFF in couples with unexplained infertility, whereas ICSI has significant benefits and results in higher cumulative pregnancy rates [8,9].
Two types of assays measure the levels of SDF: one that directly measures the extent of DNA fragmentation using probes and dyes and another that measures the susceptibility of DNA to denaturation, which is higher in fragmented DNA [7]. The acridine orange test (AOT) exemplifies the second type of assay and differentiates sperm with normal double-stranded DNA (green fluorescence) and abnormal denatured or single-stranded DNA (orange-red fluorescence) with the help of the metachromatic shift properties of the stain [10,11]. AOT is a simple and affordable test for the assessment of DNA integrity in infertile men [11,12]. Clinical assessments of SDF by AOT must be performed on the total motile fraction of sperm rather than on raw ejaculate sperm, as raw semen contains a significant number of degenerated and dead sperm with damaged DNA [13].
A study showed that couples with unexplained infertility had elevated SDF but did not explore clinical correlations with the outcomes of the ICSI cycles [4]. In previous studies, SDF values were evaluated prior to assisted reproductive treatment (ART) and, to improve outcomes, the patients were allocated to IVF or ICSI based on their SDF values [5,6,14]. It has been reported that the negative effects of SDF on clinical outcomes are attenuated in young women with high-quality oocytes [15,16]. Only fresh transfers were considered in most of the studies; thus, the cumulative live birth rate (CLBR) was not measured [4,5]. The CLBR, which includes both fresh embryo transfer (ET) and frozen embryo transfer (FET) cycles, measures the success of ART cycles [17]. This study aimed to determine the effect of SDF on the CLBR in ICSI cycles in couples with unexplained infertility.
Methods
1. Study population
We conducted a prospective study of 145 couples with unexplained infertility (median age, 30.25±4.33 years) who were undergoing their first ICSI cycles at the tertiary care center attached to our reproductive medicine unit at a medical college. This study was approved by the ethics committee of our institution. Written consent was obtained from all participating couples. A total of 145 ICSI cycles (one ICSI cycle per couple) were divided into two groups based on SDF rates: a low DNA fragmentation group (SDF ≤30%, n=97) and a high DNA fragmentation group (SDF>30%, n=48) [7,18-21]. Clinical and laboratory outcomes were correlated between the two groups.
2. Inclusion and exclusion criteria
Couples undergoing their first ICSI cycles for unexplained infertility were included in this study. The diagnosis of unexplained infertility was based on the following criteria: (1) normal ovarian reserve with an antral follicle count ≥8 and anti-Müllerian hormone levels ≥1.5 ng/mL, (2) normal tubal patency and uterine function evaluated by diagnostic laparoscopy and hysteroscopy, and (3) normal semen parameters for the male partner according to World Health Organization (WHO) 2010 criteria [22]. None of the female partners were ≥41years of age in this study population. Female partners with <5 mature metaphase II oocytes and male partners with normal semen parameters (WHO 2010 criteria) altered on the day of transvaginal oocyte recovery (TVOR) or egg collection were excluded. Participants with life-threatening diseases such as cancer or chronic kidney disease were also excluded from the study.
3. Semen analysis and preparation
Patients collected semen samples in sterile, non-toxic containers by masturbation after sexual abstinence of 2–3 days. After 30 minutes of liquefaction, samples were evaluated for count, motility, and morphology according to the WHO 2010 criteria [22]. Semen samples were prepared using two-layer density gradient centrifugation (DGC; V-GRAD 80% and 40%, Vitromed, Jena, Germany) for ICSI. SDF was evaluated on the DGC prepared semen samples.
4. Acridine orange test
The assessment of SDF was done using AOT [10]. Smears with 10 µL of post-wash samples were prepared and air-dried. Carnoy’s solution (methanol: glacial acetic acid, 3:1 vol/vol) was used to fix the slides overnight. The staining solution was prepared daily from a stock solution of acridine orange (1g/L in distilled water, stored in the dark at 4°C) at a ratio of 10 mL of stock solution to 40 mL of 0.1 M citric acid and 2.5 mL of 0.3 M sodium phosphate dibasic heptahydrate (Na2HPO4•7H2O), and the pH was adjusted to 2.5. The slides were stained with the above stain for 5 minutes, rinsed in distilled water, and covered with coverslips.
The slides were examined for SDF using a fluorescence microscope (Olympus CX31, Tokyo, Japan) under oil at ×1,000 with an excitation of 450–490 nm. Green fluorescence represented normal intact sperm, whereas red indicated fragmented and denatured sperm. Sperm with orange or yellow heads, as well as those displaying green and red colors simultaneously, were also considered fragmented [10,23]. At least 400 sperm were assessed in each slide to calculate the average SDF. Slides were fixed on the same day as semen preparation and examined the next day for SDF by AOT. A single highly skilled and trained andrologist evaluated all slides for consistency and to prevent interpersonal variability. Each stained slide was read immediately after staining to reduce variation in fluorescence intensity.
5. Ovarian stimulation
Controlled ovarian stimulation was started from day 3 of the menstrual cycle using recombinant follicle-stimulating hormone (Recagon, MSD; Gonal-F, Merck, Kenilworth, NJ, USA). A gonadotropin-releasing hormone antagonist (Cetrorelix Acetate, Emcure, Pune, India) was administered to suppress the pituitary function when a minimum of one follicle ≥14 mm was seen. Recombinant human chorionic gonadotropin (Ovidrel, Merck) was administered when three or more follicles reached a diameter of ≥17 mm and appropriate serum estradiol values were detected. TVOR was performed 35 hours after triggering with human chorionic gonadotropin.
6. ICSI procedure
The recovered oocytes were incubated in culture medium (Onestep; Vitromed) for 1–2 hours at 37°C in an atmosphere of 6% CO2, 5% O2, and the remainder N2. The oocytes were denuded by hyaluronidase (Hyadase 80 IU; Vitromed) at 37°C. The ICSI procedure, as described by Palermo et al. [24], was performed by a highly skilled embryologist. A morphologically normal and motile sperm was selected and immobilized in polyvinylpyrrolidone (PVP 7%; Vitromed). The immobilized sperm was aspirated tail-first into the injection pipette and injected into the oocyte. At 16–18 hours after ICSI, the oocytes that presented with two pronuclei and a second polar body were counted as fertilized. The fertilized zygotes were cultured until day 3 or day 5 of ICSI for ET or cryopreservation.
7. Embryo grading
According to the Istanbul consensus, day-3 embryos were graded as A, B, and C based on the blastomere number, fragmentation percentage, and multinucleation [25]. Grade A indicated a good embryo with stage-specific 6–8 blastomeres, <10% fragmentation, and no multinucleation; grade B indicated a fair embryo with stage-specific 6–8 blastomeres, 10%–25% fragmentation, and no multinucleation; and grade C indicated a poor embryo with non-stage specific blastomeres, severe fragmentation (>25%), and the presence of multinucleation.
Day-5 blastocysts were graded according to Gardner and Schoolcraft [26]. Expansion of the blastocysts was graded 3 to 6, and trophectoderm (TE) and inner cell mass (ICM) were graded as A, B, or C. Expansion of the blastocyst was graded as follows: 3, full; 4, expanded; 5, hatching; and 6, hatched. The TE was categorized as: grade A, a TE with many cells forming a cohesive epithelium; grade B, a TE with few cells forming a loose epithelium; and grade C, a TE with very few cells. Similarly, for ICM, the following grading was applied: grade A, a tightly packed ICM with many cells; grade B, a loosely grouped ICM with many cells; and grade C, an ICM with very few cells.
8. Embryo vitrification and warming
The surplus embryos were vitrified on either day 3 or day 5 by the Kitazato vitrification protocol (Kitazato, Japan) [27]. Briefly, the embryos were placed in an equilibration solution for 10–15 minutes at room temperature (RT), then transferred to a vitrification solution (VS1, VS2) for 1 minute, and later the embryos were loaded with minimum media onto the top of a vitrification device (Cryolock; Fujifilm/Irvine Scientific, Santa Ana, CA,USA). The device was plunged immediately into liquid nitrogen (LN2), capped inside the LN2, and then stored for future use.
Similarly, warming of the day-3 or day-5 embryos was done using the Kitazato thawing protocol (Kitazato) [27]. Briefly, the uncapped vitrification device from the LN2 was placed directly in a thawing solution pre-warmed to37°C and the embryos were allowed to float. After 1 minute, the embryos were transferred to a diluent solution at RT for 3 minutes and then transferred to a washing solution (WS1, WS2) for 5 minutes and 1 minute consecutively. The embryos were finally moved to a culture dish and incubated at 37°C in an atmosphere of 6% CO2, 5% O2, and the remainder N2 until the ET.
9. Endometrium preparation
After oocyte retrieval in patients undergoing ET cycles, daily micronized progesterone was administered vaginally (Crinone 8% gel, Merck, Kenilworth, NJ, USA) and on alternate days intramuscularly (Hald 100 mg, Intas, Ahmedabad, India) until the pregnancy test was confirmed negative, or continued for an additional 3 months if the pregnancy test was positive.
In FET cycle patients, oral estradiol valerate (Evadiol, Intas, Ahmedabad, India) was used in a stepwise increasing dose pattern for preparation of the endometrium. The endometrial lining and thickness were observed regularly prior to the ET. Progesterone was administered in a method like that described in the ET cycles. For a day-3 or day-5 ET, 4 or 6 days of progesterone was administered, respectively.
10. Embryo transfer
ET was performed under abdominal guided ultrasound (a maximum of 3 embryos) on either day 3 or day 5, depending on the quality of the embryos and the age of the patient. The embryos were transferred using a soft catheter (Cook, Brisbane, Australia). The serum β-hCG level was obtained 14 days after the transfer to confirm a positive pregnancy. Embryo utilization was calculated as the ratio of the number of embryos transferred and the number of embryos frozen to the total number of embryos formed. The high-quality embryo (grade A) rate at day 3 was calculated as the ratio of grade A embryos at day 3 to the total number of embryos cleaved. An intrauterine sac with the presence of a fetal heartbeat was considered a clinical pregnancy. The implantation rate was calculated as the proportion of gestational sacs determined by ultrasound to the total number of embryos transferred. Miscarriage was defined as a pregnancy loss after detection of an intrauterine pregnancy by ultrasound before 20 weeks. The CLBR was calculated as the first live birth event per woman per egg collection over 2 years.
11. Statistical analysis
Data were shown as mean±standard deviation for continuous variables and analyzed using the unpaired Student t-test. The categorical variables were presented as proportions between two groups and analyzed using the chi-square test. A stratified analysis for potentially biasing factors such as day of transfer (day 3 and day 5) and type of transfer (fresh and frozen) on the CLBR was conducted using the chi-square test. The effect of SDF on the CLBR and the modifying effects of the biasing factors were assessed using logistic regression analysis. Multivariable logistic regression was used to analyze the effect of SDF on the CLBR and miscarriage rate while adjusting for possible confounders between the positive live birth group and the negative live birth group. Sample size calculation was done using G*Power version 3.1.9.7 (Franz Faul, University of Kiel, Germany),which indicated that 138 cycles would be adequate to demonstrate a 20% proportion difference with 80% power and a 5% significance level considering the miscarriage rate as the primary outcome. A p-value of <0.05 was used to indicate statistical significance. The statistical analysis was executed using IBM SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA).
Results
1. Demographic and embryological characteristics of couples with unexplained infertility in ICSI cycles
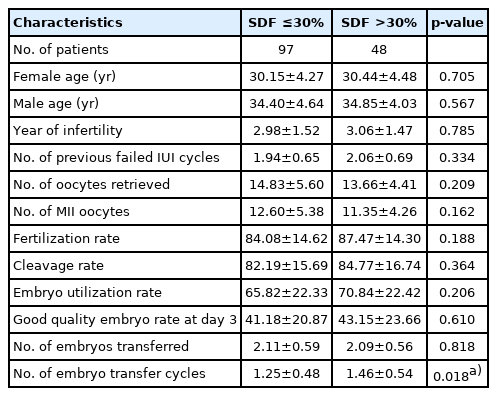
When the demographic and embryological characteristics of couples with unexplained infertility were compared between the two SDF groups (low SDF ≤30% and high SDF >30%), similar findings were observed for the ages of the female and male partners, years of infertility, number of previous failed IUI cycles, number of oocytes retrieved, number of metaphase II oocytes, fertilization rates, cleavage rates, embryo utilization rates, number of transferred embryos, and grade A embryo rates at day 3. The only meaningful difference was observed in the number of ET cycles per ICSI. A higher number of ET cycles per ICSI (p=0.018) was seen in the high SDF group compared to the low SDF group (Table 1).
2. Comparative analysis of semen parameters according to SDF group
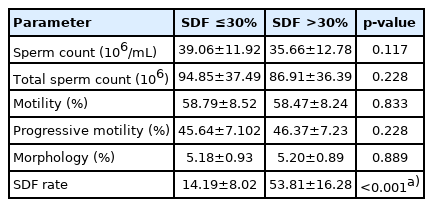
Semen parameters such as sperm count, total sperm count, motility, progressive motility, and morphology were similar between the two SDF groups, whereas a significant difference was observed in the SDF rates (p<0.001) (Table 2).
3. Clinical outcomes in patients with unexplained infertility in ICSI cycles
A total of 145 patients underwent 191 ET cycles (both ET and FET). In the low SDF group, 97 patients underwent 97 ICSI cycles and 121 ET cycles, while 48 patients in the high SDF group underwent 48 ICSI cycles and 70 ET cycles. When the clinical outcomes between the two groups were compared, the high SDF group had a significantly lower CLBR (p=0.029) and a significantly higher miscarriage rate (p=0.045) than the low SDF group (Table 3). No significant differences in the implantation rates and cumulative pregnancy rates were observed between the two groups (Table 3). The cycles were further stratified according to the type of transfer (i.e., ET or FET). In the ET cycles (n=96), 66 were in the low SDF group and 30 were in the high SDF group. The high SDF group had a significantly lower implantation rate (p=0.031), clinical pregnancy rate (p=0.005), and live birth rate (p=0.004) than the low SDF group, although there was no significant difference in the miscarriage rate. In the FET cycles (n=95), 55 were in the low SDF group and 40 were in the high SDF group, and no significant differences were found in the clinical outcomes between the groups (Table 3).
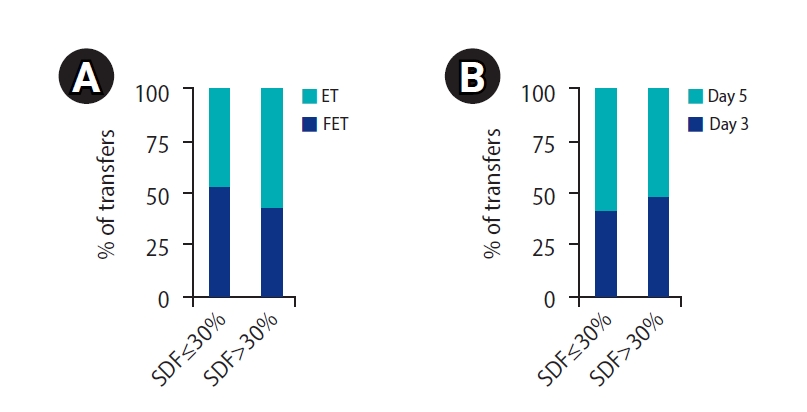
In the low SDF group, out of 121 ET cycles, 66 (54.54%) were ET cycles and 55 (45.45%) were FET cycles, whereas in the high SDF group, out of 70 ET cycles, 30 (42.85%) were ET cycles and 40 (57.14%) were FET cycles. There was no notable difference in the ET and FET cycles when the two groups were compared (p=0.119) (Figure 1). In addition, ET cycles on day 3 and day 5 were also compared between the two groups. Couples in the low SDF group underwent 50 (41.32%) day-3 and 71 (57.85%) day-5 ET cycles, and couples in the high SDF group underwent 34 (48.57%) day-3 and 36 (51.42%) day-5 ET cycles. There were no significant differences in the transfers (p=0.330) among these groups (Figure 1). A minimum of one and a maximum of three ET cycles were done per ICSI.
4. Stratification of biasing factors and their effect on the CLBR
The day of transfer (day3/day5) and type of transfer (fresh/frozen) were considered as biasing factors. There was no significant difference in the live birth rate of the day-3 or day-5 transfers (p=0.145). Similarly, there was no significant difference in the live birth rate between the fresh and frozen transfer cycles (p=0.494) (Table 4). The biasing factors did not modify the effect of SDF as an independent predictor of cumulative live birth (odds ratio [OR], 0.986; 95% confidence interval [CI], 0.971–1.001; p=0.071) when evaluated using logistic regression analysis. The day of transfer did not modify the effect of SDF on the probability of cumulative live birth (OR, 0.987; 95% CI, 0.972–1.002; p=0.095) and the type of transfer also did not modify the effect of SDF on the probability of cumulative live birth (OR, 0.986; 95% CI, 0.971–1.002; p=0.083) (Table 4).
5. Demographic and embryological characteristics of couples with unexplained infertility in live birth groups
The couples with unexplained infertility were divided into two groups based on live birth outcomes: (1) the positive live birth group and (2) the negative live birth group. These two groups showed significant differences in the ages of both male (p=0.020) and female partners (p=0.034), the embryo utilization rate (p=0.023), and grade A embryos (p=0.045). No remarkable difference was noted in the SDF rates, number of mature oocytes, fertilization rates, cleavage rates, number of embryos transferred, and number of ET cycles per ICSI between the two groups (Table 5).
6. SDF as a predictor of cumulative live birth and miscarriage in the ICSI cycles of couples with unexplained infertility
When adjusted for the possible confounders between the positive live birth and negative live birth groups, multivariate logistic regression analysis showed that SDF was a predictor of cumulative live birth in couples with unexplained infertility (p=0.047), although it did not significantly predict miscarriage (p=0.621) (Table 6).
Discussion
Routine semen analysis plays a salient role in the infertility evaluation of men. However, its role is minor for couples with unexplained infertility since sperm abnormalities at the DNA level cannot be identified by routine methods. SDF, rather than normal semen analysis, has good diagnostic and prognostic capabilities for men with idiopathic infertility based on routine semen parameters [28-30].
SDF can occur pre- or post-ejaculation due to various mechanisms, as described by Sakkas and Alvarez [31] and others [32]. The integrity of sperm DNA is necessary for proper fertilization and embryo development. A study suggested that early paternal effects, before embryonic genome activation, were not related to SDF, but that SDF was related to late paternal effects and could increase the risk of miscarriage [33]. Other studies also determined that the effect of SDF on pregnancy rates was modest in IVF cycles and had slight to no effect in ICSI cycles [4,7,34-37]. In contrast, some studies reported the negative effect of SDF on pregnancy rates in ICSI cycles [21,38,39]. In most studies, the correlation of SDF with clinical outcomes was limited to pregnancy rates only. According to a recent meta-analysis, very few studies correlated SDF with live birth rates in cycles of ICSI [14] and even fewer correlated SDF with CLBR. This is the first study to correlate SDF with CLBR in couples with unexplained infertility undergoing ICSI cycles. The data on CLBRs provided by this study are particularly significant because both fresh and frozen ET outcomes were included in the analysis. It is challenging to report the CLBR, as the definition of this rate is inconsistent. In our study, the CLBR was defined as the first live birth event achieved from one TVOR/egg collection cycle over a period of 2 years [40]. All patients in the present study underwent at least one ET cycle after TVOR.
The main outcome measure of the present study was the CLBR in correlation with SDF in couples with unexplained infertility in ICSI cycles. The high SDF group had a 1.5-fold lower CLBR (p=0.029) (Table 3) and a 2.0-fold higher miscarriage rate (p=0.045) (Table 3) than the low SDF group. In this study, SDF was not correlated with fertilization, cumulative pregnancy, and implantation rates, but there was a trend for high SDF to be associated with a lower implantation rate (p=0.063) (Table 3), which was also observed in other studies [7,41]. In the stratification of transfer cycles, ET cycles had significant differences in the clinical outcomes between the high and low SDF groups (Table 3), whereas in FET cycles, clinical outcomes were similar between the high and low SDF groups, probably because some patients underwent more than one FET cycle and tended to opt for the maximum number of embryos to be transferred (i.e., 3) due to previous failed cycles. Nonetheless, the analysis of all cycles showed significant associations between SDF and the live birth rate and miscarriage rates. Other studies have also found lower rates of implantation, clinical pregnancy, and live birth in ET cycles [4,7,41]. As reported in other studies, we also found no remarkable differences in the grade A embryo rate at day 3 in both SDF groups after ICSI cycles [34,35,37]. In contrast, some studies contradicted these results and reported poor quality embryo outcomes in the high SDF group [7,36]. In most studies, the embryo utilization rate was not mentioned because only ET cycles were considered [4,5,7].
ICSI has been the most favored method for treating couples with well-defined idiopathic infertility [8]. It was evident from a previous study that couples can achieve a higher take-home baby rate with ICSI cycles rather than with conventional IVF cycles [42]. Therefore, in this study, all couples underwent ICSI irrespective of the SDF percentage. In most studies, SDF was evaluated prior to the ART cycles and IVF or ICSI cycles were chosen based on the SDF values, or samples were frozen and/or evaluated when needed [5,41,43,44]. In this study, the SDF was evaluated in the actual sperm to be used for the ICSI cycles and clinically correlated in an unbiased manner to improve the outcome.
The negative correlation of SDF with the live birth rate in IVF cycles was established in a recent meta-analysis [14] where the pooled data of six studies identified a negative correlation between SDF and live birth rates in ICSI cycles. However, the detrimental effect was nullified in a sub-group analysis that only included studies with female factors (age and ovarian reserve). Further studies on this issue are needed [14].
One study reported a significant difference in the live birth rate in IVF cycles with a high SDF and, to a lesser degree, in ICSI cycles; the weaker findings in ICSI cycles can be explained by the fact that there were many fewer patients in the low SDF group (<25%). The same study showed an approximately 12% lower live birth rate in ICSI patients with SDF of 25%–50% compared to those with SDF >50% [4]. Similarly, other studies stated that couples in the high SDF groups had lower rates of ongoing pregnancy in ICSI cycles, which was corroborated by the present study [41,43]. In contrast, other studies reported no significant correlation between the live birth rate and SDF in either IVF or ICSI cycles [44].
SDF was positively correlated with the miscarriage rate in this study at a threshold of 30%. Spontaneous abortion rates were higher in ICSI cycles with SDF>30%, as reported by Zini et al. [36]. In a meta-analysis by Robinson et al. [45], a review of 16 studies and other recent studies corroborated that SDF was positively correlated with spontaneous miscarriage [7,46]. Even with optimizations such as semen sample preparation by DGC, morphologically good sperm selection through ICSI, and the selection of high-quality embryos for transfer, the miscarriage rates were significant when correlated with SDF in this study. As mentioned earlier, this may be attributed to the late paternal effect of male gene expression [33].
To some extent, the effect of SDF on the clinical outcome depends on the quality of the oocyte [15]. Sperm depends on the oocyte for post-fertilization DNA repair, and high-quality oocytes can help mitigate the effect of SDF on pregnancy outcomes [15]. The female partners were significantly younger in the positive live birth group (29.56 years) than in the negative live birth group (31.08 years, p=0.034) (Table 5). Growing evidence suggests that high-quality oocytes from younger women can overcome the effect of SDF on pregnancy outcomes [15,16], as corroborated by the present study.
The SDF, fertilization, and cleavage rates showed no notable differences between the live birth groups. In the positive live birth group, the grade A embryo rate was higher (p=0.045) (Table 5), which led to a higher embryo utilization rate (p=0.023) (Table 5). This may be attributed to the young female partners with high-quality oocytes in the positive live birth group as compared to the negative live birth group, whose female partners were comparatively older. High-quality oocytes have the capacity to repair damaged sperm DNA even despite SDF. Similar conclusions have been proposed in other studies [15]. Since female age, embryo utilization rate, and the grade A embryo rate showed statistically significant differences between the live birth groups, they were considered as confounding factors. When the effect of SDF on the cumulative live birth was adjusted for these confounding factors, SDF was a significant predictor of cumulative live birth (p=0.047) (Table 6) in the ICSI cycles of couples with unexplained infertility. The effect of SDF on the CLBR was not significant (p>0.05) (Table 4) when modified by these biasing factors; therefore, they were not considered as confounding factors for the CLBR in couples with unexplained infertility.
AOT is an established method for assessing the integrity of sperm DNA in infertile men [11,12]. Using AOT, an unfavorable effect of SDF on pregnancy and implantation rates was found in the high SDF group in ICSI cycles [21], and this finding has clinical significance for patients with repeated early pregnancy loss [46]. The miscarriage rate was directly correlated with SDF in this study. Of the 16 studies included in a meta-analysis on SDF and miscarriage, eight used AOT, six used the TdT (terminal deoxynucleotidyl transferase)-mediated dUDP nick-end (TUNEL) assay, and two used the comet assay [45].
The AOT method is simple, inexpensive, and convenient to use routinely in-house. The principle of AOT is similar to that of the sperm chromatin structural assay (SCSA) except for the number of sperm counted. In this study, a trained and technically skilled in-house embryologist evaluated the slides for SDF. We have been using the AOT method to assess SDF since 2012 for various research projects [47]. Although the AOT is not as robust as the SCSA, the cells can be differentiated easily, and the SDF rate can be evaluated technically. However, there is a lack of consistency across studies regarding the threshold value for AOT, which is set at 30%–50% for clinical correlations [12,21,48]. In this study, at a threshold value of 30%, the SDF was inversely correlated with the CLBR and directly correlated with the miscarriage rate of ICSI cycles in couples with unexplained infertility.
The percentage of couples with high SDF in this study was approximately 33% of all couples with unexplained infertility. The low percentage compared to other studies may have been due to the use of prepared sperm samples to evaluate SDF rather than raw semen samples [4,5]. Although ICSI was performed in order to optimize outcomes in all couples, the couples with high SDF needed to undergo a significantly higher number of ET cycles (p=0.018) (Table 1) than the low SDF group, which is both financially and emotionally burdensome to couples. Furthermore, even with more ET cycles, the fertility rate was significantly lower in the high SDF group than in the low SDF group. After a negative result, many couples do not return for another transfer even if they have embryos frozen. Therefore, treatment interventions to reduce the SDF such as antioxidant therapy, lifestyle modifications, and dietary supplements [49-52], or the use of techniques (e.g., microfluidics and magnetic-activated cell sorters) to select sperm with low or barely detectable levels of SDF without further damaging the sperm cells can be used to improve the clinical outcomes [53,54].
Despite the valuable results obtained in the study, the authors recognize its limitations. The sample size was small because only couples with unexplained infertility who underwent ICSI cycles were included. The AOT method may not be as robust as the gold-standard SCSA method but, as already mentioned, the AOT method is simple, inexpensive, and comparable to the SCSA method. We were unable to calculate the blastulation rate as some patients underwent both day-3 and day-5 ET cycles. Finally, SDF is a contributing factor along with other confounders, not an independent predictor of CLBR in the ICSI cycles of couples with unexplained infertility.
In conclusion, SDF negatively influenced the CLBR, and a high SDF was associated with a higher miscarriage rate in the ICSI cycles of couples with unexplained infertility. These findings suggest that there is a need to evaluate SDF prior to ART cycles in couples with unexplained infertility to enable better counseling.
Acknowledgements
We would like to acknowledge all staff members of the reproductive medicine and surgery department and the couples who participated in the study.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: DR. Data curation: DR. Formal analysis: DR. Methodology: DR. Project administration: DR, SB. Visualization: DR, KVRS. Writing–original draft: DR. Writing–review & editing: all authors.