Effect of aqueous Nigella sativa extract on the functional parameters of post-thaw human spermatozoa during vitrification
Article information
Abstract
Objective
Sperm vitrification leads to the production of reactive oxygen species (ROS) that can damage the functional parameters of sperm. The present study aimed to investigate the antioxidant effect of Nigella sativa extract on motility, plasma membrane function, mitochondrial membrane potential (MMP), DNA damage, and intracellular ROS production.
Methods
A total of 20 sperm samples were used. Samples were divided into six experimental groups, including groups with aqueous extract from N. sativa seeds at concentrations of 1% to 6%, a cryopreserved control group, and a fresh control group.
Results
Statistical analysis showed significantly higher total sperm motility at concentrations of 3% to 6% than in the vitrified semen control group. Additionally, progressive motility and all motion characteristics at all concentrations were significantly higher than in the vitrified semen control group. The presence of N. sativa seed extract also improved the quality of the sperm parameters assayed in all experimental groups (1%–6%; intracellular ROS production, DNA damage, MMP, and sperm membrane function) compared to the control group.
Conclusion
Higher concentrations of N. sativa led to improvements in all sperm parameters and sperm quality. These findings indicate that N. sativa seed extract is effective for improving the quality of sperm after vitrification.
Introduction
The process of sperm cryopreservation plays an important role in artificial insemination. Although artificial insemination using fresh sperm tends to be more successful, the fertility rate decreases when cryopreserved sperm is used [1]. During cryopreservation, large amounts of reactive oxygen species (ROS) are produced, resulting in increased lipid peroxidation. The sperm plasma membrane contains high amounts of unsaturated fatty acids that are oxidized by ROS [2]. The cryopreservation process can cause intracellular ice crystallization, osmotic stress, and cold shock, which could ultimately negatively affect sperm structure and function [3]. Oxidative stress products can affect sperm characteristics such as motility, cell membrane potential, mitochondrial membrane potential (MMP), and DNA activity, disrupting sperm function and fertility [4]. Cryopreservation can be undertaken using two methods: slow freezing and vitrification. While slow freezing increases damage to sperm and tissue, it has been the most conventional and common cryopreservation method for many years. Vitrification tends to be used as an alternative method to slow freezing. Vitrification is a relatively new method for sperm cryopreservation that has advantages over conventional methods, such as reducing intracellular ice crystals. Moreover, compared to slow freezing, vitrification is cheaper and freezes sperm in less time [5]. Sperm naturally contains many antioxidant systems capable of reducing intracellular damage [6]. An antioxidant system in the seminal plasma consists of the enzymatic antioxidants, catalase, and superoxide dismutase (SOD), with its two isozymes playing a critical role in eliminating ROS production. SOD preserves the quality of spermatozoa by reducing lipid peroxidation and oxygen toxicity [7]. The presence of SOD and catalase reduces lipid peroxidation by removing the superoxide anion produced by reduced nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase in neutrophils [8]. Glutathione peroxidase, which is another important enzymatic antioxidant, removes peroxyl from hydrogen peroxide. Moreover, natural antioxidants such as vitamin E, pyruvate, urate, ascorbate, glutathione, vitamin A, albumin, ubiquinol, taurine, hypotaurine, vitamin C, beta-carotenes, and carotenoids are non-enzymatic antioxidants present in seminal plasma [9]. The vitrification process reduces the antioxidant protection of the sperm membrane [10]. However, an imbalance between oxidative stress products and the antioxidant system of sperm during the vitrification process is the principal cause of cryopreservation damage [11,12].
Many recent studies have been conducted on the use of antioxidants to reduce the damaging effects of the cryopreservation process. Nigella sativa is a medicinal plant known as black seed, black cumin, or shouneez that has several therapeutic effects on different diseases. For instance, a study showed that N. sativa oil improved the sperm count, motility, morphology, semen volume, and pH of raw semen in infertile men [13]. Another study showed that N. sativa improves the raw sperm parameters and acrosomal function of cyclophosphamide-induced testis toxicity in mice [14]. It was reported that N. sativa extract (NSE) at all concentrations (1%–6%) improved the quality of buffalo sperm that had been damaged in the vitrification process by an antioxidant mechanism [15]. N. sativa seeds contain carbohydrates, eight of the nine essential amino acids, vitamins, minerals, and antioxidants [16]. Various sources have reported the nutritional composition of N. sativa (20%–85% of protein, 38.20% of fat, 7%–94% of fiber, and 31.94% of total carbohydrates). Glutamate, arginine, aspartate, cysteine, and methionine are the major and minor amino acids,. Black cumin seeds also contain iron, copper, zinc, phosphorus, calcium, thiamine, niacin, pyridoxine, and folic acid [17]. N. sativa has been proven to have antifungal and antibacterial effects, as well as antioxidant properties [18]. N. sativa is believed to contain essential elements such as thymoquinone, dithymoquinone, 4-terpineol, carvacrol, anethole, thymol, and alpha-pinene [15]. Evaluation of N. sativa oil using thin-layer chromatography showed that thymoquinone, carvacrol, and 4-terpineol have a very strong antioxidant effect on the sperm parameters of vitrified buffalo sperm [15]. Although studies conducted on rabbits, mice, and humans have shown that NSE improves the quality of raw sperm parameters [19-21], no study has yet been conducted on the antioxidant effect of aqueous NSE on the functional parameters of human sperm during the vitrification process. The main hypothesis of this study was that NSE, which reduces ROS production in sperm due to its antioxidant mechanism, improves the quality of vitrified semen. Therefore, this study aimed to evaluate the antioxidant properties of aqueous NSE on motility, plasma membrane integrity, MMP, DNA damage, and intracellular ROS production in human sperm.
Method
1. Subjects and semen collection
This study was conducted at the Research Center and Laboratory of Tehran University of Medical Sciences using 20 normal sperm samples obtained from men referred to the Aban Infertility Center from February 2020 to April 2020. Samples were obtained from patients via masturbation after 3 to 7 days of abstinence from sex. This study was approved by the ethics committee of Tehran University of Medical Sciences (IR.TUMS.MEDICINE.REC.1398.887). Written informed constant was obtained from participants. The sperm parameters were assessed according to World Health Organization standards (2010), and sperm motility and concentration were assessed using the SCA CASA system (Sperm Class Analyzer ver. 5.1; Microptic S.L., Barcelona, Spain). Only sperm samples with a concentration of more than 1×106 sperm/mL, progressive motility of 70%, and a volume of 2 mL to 6 mL were used for this study.
2. NSE preparation
N. sativa seeds were prepared by a plant classification expert from a local medicinal plant market. The aqueous extract was prepared based on the protocols used in previous studies with similar backgrounds [15]. N. sativa seeds were crushed after being washed with distilled water and dried at 50°C. Next, 10 g of seed powder was mixed with 50 mL of distilled water for 15 to 20 minutes. The mixture then rested for 30 minutes. After centrifugation (1,340 ×g, 15 minutes), the supernatant was separated using Whatman filter paper. The filtration was performed twice to increase accuracy. The extract sterilization was carried out using Acrodisc (Merck, Kenilworth, NJ, USA), and the extract was stored in special sterile containers at 4°C for several days until use. Finally, the intended concentrations of the extract were prepared based on previous studies [15]. Concentrations of 1% containing 30 µL of extract and 170 µL of distilled water, 2% containing 60 µL of extract and 140 µL of distilled water, 3% containing 90 µL of extract and 110 µL of distilled water, 4% containing µL 120 of extract and 80 µL of distilled water, 5% containing 150 µL of extract and 50 µL of distilled water, and 6% containing 180 µL of extract and 20 µL of distilled water were prepared [15].
3. Vitrification and warming
The micro-droplet technique was used for the vitrification method [22]. In this process, samples were first mixed with human tubal fluid (HTF; Sigma-Aldrich, St. Louis, MO, USA) solution and then added to a solution containing 5% human serum albumin (Sigma-Aldrich) and 0.5 mol/L sucrose. Next, the sperm solution was divided equally into six experimental groups, and concentrations of 1% to 6% extract were added to each of the groups, respectively. No NSE was added to the solution for the raw semen control group. Finally, 30 µL of the prepared suspension was incubated in a tank containing liquid nitrogen for 1 week. At the thawing stage, the samples and 5 mL of the preheated HTF solution at 37°C were first mixed with 1% HAS, and, after incubation, the suspension was kept at 37°C near 5% CO2 for 5 minutes. Finally, the samples were centrifuged at 400 ×g for 5 minutes and suspended in 50 µL of HTF [4].
4. Assessment of sperm motion characteristics
In this assessment, a 10-µL sperm sample was first placed on a pre-heated Makler slide at 37°C and examined using the CASA system. The aforementioned parameters, including total motility (%), progressive motility (%), average path velocity (VAP; µm/sec), curvilinear velocity (VCL; µm/sec), linearity (LIN; %), and straight-line velocity (VSL; µm/sec), were examined. Five microscopic fields and 400 total spermatozoa were examined.
5. Assessment of functional sperm membrane
To investigate the sperm plasma membrane function, the hypo-osmotic solution test was used. To prepare the solution, 0.73 g of sodium citrate and 1.35 g of fructose (Merck) were dissolved in 100 mL of distilled water (osmolality ~190 mOsmol/kg). Finally, 50-µL sperm samples were dissolved in 500 µL of HOS solution (at 37°C for 45 minutes). Next, 10 µL of this solution was transferred to a slide, and the samples were evaluated using phase-contrast microscopy (Olympus BX20, Olympus, Tokyo, Japan) [23].
6. Assessment of mitochondrial membrane plasma
Lipophilic cationic dye, JC-1 (T4069, Sigma-Aldrich), was used to investigate MMP. After being centrifuged (500 ×g, 5 minutes), the samples were dissolved in phosphate-buffered saline (PBS) until a sperm concentration of 1×106 sperm/mL was obtained. Next, 1 µL of JC-1 dye was added to 1 mL of this suspension. Finally, flow cytometry was used. In this technique, red fluorescence and orange fluorescence were detected using FL1 (530 nm) and FL2 (585 nm) detectors, respectively [24].
7. Assessment of DNA damage
Acridine orange fluorescence was used to determine DNA damage. The centrifuged samples (500 ×g, 5 minutes) were added to a Tris-null EDTA (ethylenediaminetetraacetic acid) buffer solution containing 1 mmol EDTA, 10 mmol Tris, and 0.15 mol NaCl. Using this technique, green fluorescence, indicating normal chromatin, and red fluorescence, indicating abnormal chromatin, were detected using FL1 (500-530 nm) and FL2 (620 nm) detectors, respectively [25].
8. Assessment of intracellular ROS production
Dihydroethidium (DHE) was used to measure intracellular ROS production. DHE is oxidized by the superoxide anion and binds to DNA, and it produces red fluorescence. The samples were suspended in PBS solution after being thawed at a concentration of 1×106 sperm/mL. Next,10 µL of DHE solution (Sigma-Aldrich) was added to the previous solution and kept at 25°C for 20 minutes. Finally, the flow cytometry technique was used. With this technique, red fluorescence was detected using FL2 (525–625 nm) [23].
9. Flow cytometry analysis
Flowcytometric analysis of the sperm parameters was conducted using FACSCaliber (BD Biosciences, San Jose, CA, USA). The samples were excited using an argon laser at 488 nm. SYBR‐14 and propidium iodide (PI; Molecular Probes, Eugene, OR, USA) were dissolved in anhydrous DMSO (4 μM) and distilled water (1 mM), respectively. Aliquots of the two working solutions were mixed at a 1:1 ratio, and 10 μM was added per counting tube, resulting in a concentration of 50 nM SYBR‐14 and 12 μM PI. Three events were assessed using this technique, which was replicated three times. Gating settings for the sperm population (gated events) used to exclude non-sperm events were based on Hoechst 33342 staining. A total of 10,000 spermatozoa were assessed using flow cytometry and analyzed using Cyflogic (ver. 1.2.1; CyFlo Ltd., Turku, Finland).
10. Statistical analysis
All data were analyzed using the Kolmogorov Smirnov test to confirm a normal distribution of values. One-way analysis of variance and the Tukey test were used to determine differences between the various groups. Statistical analysis was performed using SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA). The results are shown as means with standard errors. Differences were considered to be statistically significant at p<0.05.
Results
1. Effects of NSE on sperm parameters (total motility, progressive motility, and motion characteristics)
Vitrification caused a significant decrease in the percentage of total motility, progressive motility, and motion characteristics (VSL, VCL, LIN, and VAP) compared to the raw semen control group (p<0.05). No significant difference was observed in total motility in the groups containing 1% and 2% concentrations compared to the vitrified semen control group, while the groups with 3% to 6% concentrations preserved the quality of sperm better compared to the control group (p≤0.05). The addition of the NSE at concentrations of 1% to 6% also improved the percentage of progressive motility and motion characteristics (VSL, VCL, LIN, and VAP) compared to the control group (p≤0.05) (Table 1).
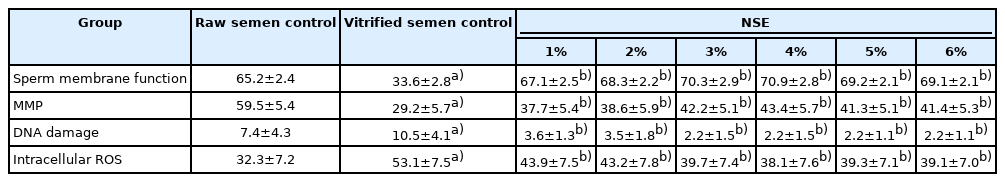
2. Effects of NSE on sperm membrane function, MMP, DNA damage, and intracellular ROS production
Vitrification resulted in a noticeable decrease in sperm membrane function and MMP and an increase in intracellular ROS production and DNA damage compared to the raw semen control group (p<0.05). The presence of NSE at all 6 concentration levels caused a significant increase in sperm membrane function and MMP compared to the vitrified semen control group (p≤0.05), while no significant increase was observed compared to the raw semen control group. Moreover, all concentrations of NSE led to a significant reduction in intracellular ROS production and DNA damage compared to that of the vitrified semen control group (p≤0.05) (Table 2).
Discussion
The present study investigated the effect of different concentrations of aqueous NSE on the parameters of human sperm during vitrification. This study’s findings revealed that the vitrification process significantly damaged sperm quality, which was assessed according to their percentage of total motility and motion parameters. NSE, as a tool for sperm cryoprotection, preserved the normal quality of sperm parameters. Although NSE improved progressive motility and motion parameters at all concentrations, the percentage of total motility improved the most at concentrations of 3% to 6%. Moreover, while other variables evaluated in this study were also affected by the vitrification technique, the presence of NSE reversed the effects of vitrification. The quality of the functional sperm membrane and MMP decreased and intracellular ROS production increased in the vitrified semen control group compared to the raw semen control group. NSE improved the above parameters at all concentrations.
The ROS produced during the cryopreservation process have an adverse effect on sperm structure and function. Therefore, the production of ROS should be minimized to improve sperm function during cryopreservation. However, multiple studies have shown that the cryopreservation process reduces sperm antioxidants [26,27]. In addition, studies have proven that reduced antioxidant activity leads to oxidative stress reactions that disrupt the sperm parameters, such as sperm motility, plasma membrane integrity, and DNA [4]. As a medicinal plant, N. sativa has strong antioxidant properties. This property is due to the presence of effective compounds such as thymoquinone, thymol, and dithymoquinone. Vitamin E, a potent antioxidant, is also one of the components present in the seeds of N. sativa [28]. N. sativa contains elements such as iron, sodium, potassium, and copper that increase sperm maturity and can also be cofactors for several enzymes that protect semen [29]. The antioxidant properties of thymoquinone include the ability to eliminate hydroxyl and superoxide radicals [30]. The antioxidant properties of thymoquinone may depend on the redox property of the quinine structure of the thymoquinone molecule that causes it to pass through morphological barriers and penetrate intracellular spaces, reducing ROS production. The thymoquinone present in NSE can increase non-enzymatic antioxidant activity such as glutathione and, in addition to spontaneous reaction with glutathione, NADH, and NADPH, produce species such as glutathione and dihydrothymoquinone that protect sperm from oxidative stress damage [31]. The results of this study confirm the protective properties of N. sativa against oxidative stress. In the groups containing NSE at all concentrations (1%–6%), a significant decrease in intracellular ROS production was observed. The extent of the reduction increased according to the concentration of NSE, from 1% to 6%, compared to the vitrified semen control group [15]. A previous study found that NSE was capable of controlling free radicals, such that the antioxidant activity of NSE increased from 1% to 6%, and this result is in agreement with that of our study. Motility is one of the main characteristics of sperm related to successful fertility. Sperm with normal motility can reach the location of the egg and result in normal fertility. Therefore, an understanding of sperm motility is very important in research studies.
Our results showed an upward trend in sperm motility at concentrations of 1% to 4% compared to the cryopreserved control group. While this increase was observed at low concentrations of 1% to 4%, at higher concentrations of 5% and 6%, motility began to decrease. Kolahdooz et al. [13] showed that N. sativa oil improved sperm parameters (count, morphology, and motility) in infertile men. Supplementation with N. sativa oil in the vitrified semen of goats showed that N. sativa could preserve the quality of sperm parameters and the sperm membrane [32]. N. sativa oil has been reported to improve sperm parameters, testosterone level, and MMP in rats exposed to an obesogenic diet [33]. A study conducted in 2018 showed that aqueous NSE in cryopreserved buffalo spermatozoa improved sperm parameters, DNA integrity, and plasma membrane integrity due to its antioxidant activity, and these results are similar to those of this study [15]. Increased sperm motility at concentrations of 1% to 4% and decreased sperm motility at concentrations above 4% are likely due to thymoquinone. Thymoquinone has antioxidant properties at low concentrations but causes an increase in ROS production at concentrations higher than 4%, which leads to a reduction in motility [34,35]. The results related to sperm motility also hold true for the results of motion characteristics (VSL, VCL, LIN, VAP). ROS are produced in mitochondria during the oxidative phosphorylation process. In the experimental groups, NSE at concentrations of 1% to 6% showed a significant increase in MMP compared to the vitrified semen control group and this increase exhibited an upward trend up to a concentration of 4% according to previous results. This increase in MMP was probably caused by a reduction in intracellular ROS production due to the antioxidant properties of NSE. Previous studies have observed improvements in the sperm quality of humans and different animal species due to the presence of NSE [20,21]. Our assessment of sperm DNA damage showed that aqueous NSE reduced DNA damage in the groups with concentrations of 1% to 6% compared to that in the vitrified semen control group and the raw semen control group. This decrease can be attributed to the effects of NSE on DNA structure and density. These results are in agreement with the findings of previous studies showing that NSE supplementation in extenders improved DNA integrity [36].
The presence of aqueous NSE during the sperm vitrification process protects sperm against oxidative stress and reduces ROS production. This reduction can improve sperm parameters, such as motility, plasma membrane function, MMP, and DNA damage. This property can be attributed to the robust antioxidant activity of the constituent elements of NSE. Although further research is needed on the effects of plant extracts on sperm function, given the available evidence, NSE can be suggested as a powerful and inexpensive antioxidant to improve vitrification conditions.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: AS. Data curation: ZN, FG. Formal analysis: MS. Acquisition: MS. Methodology: MS, AS. Project administration: FG. Visualization: MS. Writing–original draft: FG, AS. Writing–review & editing: ZN.