The effect of lipopolysaccharide from uropathogenic Escherichia coli on the immune system, testis tissue, and spermatozoa of BALB/c mice
Article information
Abstract
Objective
Uropathogenic Escherichia coli is known to cause urinary tract infections, and the endotoxin (lipopolysaccharide [LPS]) of this bacterium may cause deficiencies of sperm quality and morphology. In the present study, the effects of LPS on mouse sperm were studied, and the levels of interleukin (IL)-17A and possible changes in testis tissue were evaluated.
Methods
LPS of uropathogenic E. coli was extracted using the methanol-chloroform method, followed confirmation using sodium dodecyl sulfate-polyacrylamide electrophoresis. Purified LPS (100 µg/kg) or phosphate-buffered saline was injected intraperitoneally into BALB/c mice for 7 days consecutively in the test and control groups, mice were sacrificed on days 3, 7, and 42 after the first injection. Blood was tested for levels of IL-17A using the enzyme-linked immunosorbent assay method. Testis tissue and sperm were collected from each mouse and were studied according to standard protocols.
Results
The mean sperm count and motility significantly decreased (p=0.03) at 3, 7, and 42 days after the injections. The level of IL-17A in the test groups increased, but not significantly (p=0.8, p=0.11, and p=0.15, respectively). Microscopic studies showed no obvious changes in the morphology of the testis tissue; however, significant changes were observed in the cellular parenchyma on day 42.
Conclusion
LPS can stimulate the immune system to produce proinflammatory cytokines, resulting in an immune response in the testis and ultimately leading to deficiency in sperm parameters and testis tissue damage. In addition, the presence of LPS could significantly impair sperm parameters, as shown by the finding of decreased motility.
Introduction
According to currently available data, 15% of cases of male infertility are due to sexually transmitted infections (STIs) [1,2]. Both Gram-positive and Gram-negative bacteria are associated with STIs, and these infections may cause men to become infertile [3]. Among these bacterial agents, uropathogenic Escherichia coli has been recognized as a Gram-negative bacterium that plays an important role in male infertility, and data have shown that the most common microbe isolated from semen was E. coli [4,5].
E. coli can damage spermatozoa through various mechanisms, including one-way direct interactions between bacteria and spermatozoa that result in immobilization of spermatozoa [6]. The tight adhesions between bacteria and sperm cause sperm to become immobile and to agglutinate, resulting in dramatic structural alterations and damage. Another mechanism is soluble factors produced and secreted by pathogenic bacteria [7,8]. For instance, the E. coli endotoxin (lipopolysaccharide [LPS]) can lead to the loss of sperm viability and sperm DNA fragmentation [9,10]. Furthermore, proinflammatory cytokines, which are usually released by leukocytes during the inflammatory response, impair sperm quality [11]. Interleukin (IL)-17 is a proinflammatory cytokine important for host immune modulation in infection and inflammatory diseases. IL-17A has been shown to play a critical role in bacterial infections [12,13]. Although IL-17A seems to be unnecessary for the generation of a protective response to uropathogenic E. coli, it may nonetheless cause adverse effects on spermatozoa. In this study, the effects of LPS extracted from E. coli and the subsequent production of IL-17A on both the quality and quantity of mouse spermatozoa and testis tissue were investigated in comparison with control mice.
Methods
1. Ethics statement
The present study was approved by the Ethical Committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran (No. IR.SSU.MEDICINE.REC.1394.263). All animal testing procedures were performed in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals [14].
2. Bacterial purification
Uropathogenic E. coli ATCC 7852 containing the eaeA gene was inoculated on an eosin methylene blue medium (Merck, Darmstadt, Germany), and following incubation at 37°C for 24 hours, the grown colonies were further mixed with 250 mL of Luria-Bertani broth medium (Merck). After overnight incubation at 37°C, the mixture was centrifuged (180 rpm). Then the supernatant was discarded and the sediment was mixed with 95% alcohol. The process followed the instructions in the manual published by Sezonov et al. [15].
3. LPS extraction by chloroform methanol
Dried bacteria were suspended in 1 mL of 10% ethylenediaminetetraacetic acid (EDTA) solution and then sonicated for 20 minutes. One milliliter of methanol/chloroform (1:2 ratio) in a saturated solution was added to the bacterium-EDTA solution and the tube lids were closed and shaken for 2 hours, then centrifuged at 2,000 rpm for 10 minutes. After the formation of three layers (methanol, cellular extract, and chloroform, respectively) the chloroform and methanol layers were separated, and after evaporation, the bacterial LPS was obtained [16].
4. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis analysis
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis was carried out to confirm the extracted LPS in comparison to commercially available LPS. The extracted LPS was dissolved into 0.05 M Tris-HCl buffer with a pH of 6.8 containing 2% SDS, 10% sucrose, and 0.01% bromophenol blue, and incubated at 100°C for 5 minutes. Then, 0.5 µg of extracted LPS and commercial LPS (Sigma-Aldrich, St. Louis, MA, USA) were loaded into the polyacrylamide gels. For detection of the bands, the gel was visualized with the silver staining method [17,18].
5. Intraperitoneal injection of LPS extracted in mice (BALB/c)
Twenty-eight healthy adult male BALB/c mice with an average age of 7–8 weeks and homogeneous weight were obtained from the animal laboratory facilities at Shahid Sadoughi University of Medical Sciences. All experimental animals were housed in standard environmental conditions in cages maintained at an ambient temperature of 25°C±2°C under 12-hour light/dark conditions. The animals had free access to food and water during the experiment. The mice were randomly divided into four groups (group 1, control; group 2, test sample on day 3; group 3, test sample on day 7; and group 4, test sample on day 42). All mice received intraperitoneal injections for 7 days with phosphate-buffered saline (PBS) in the control group and 100 μg of isolated LPS per kilogram of body weight per day in the test group. After 3, 7, or 42 days, the mice were sacrificed by cervical dislocation. The cauda epididymis of each animal was dissected and placed in 1 mL of Ham’s F10 medium and then incubated for 30 minutes [19].
Sperm parameters including total sperm count, normal morphology, and motility were evaluated for each mouse. For motility and count, a Makler chamber (Sefi Medical Co., Haifa, Israel) and phase contrast microscopy were used (Olympus, Tokyo, Japan) at ×20. The motility of sperm was graded as rapid linear motile (A), slow linear (B), nonprogressive (C), and immotile (D). Sperm morphology was evaluated by bright field microscopy and Giemsa staining. All analyses were done by an experienced technician according to World Health Organization criteria [20]. Blood samples were also collected from each mouse by cardiac puncture. The serum was then separated and the level of IL-17A was measured using a commercial enzyme-linked immunosorbent assay kit (eBioscience, San Diego, CA, USA) according to the kit’s protocol.
6. Statistical analysis
Between-group comparisons were performed using the nonparametric Mann-Whitney U-test. All statistical analyses were performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA) and p-values of less than 0.05 were considered to indicate statistical significance.
Results
SDS-PAGE analysis and silver staining showed that the extracted LPS had a similar molecular weight to that of the commercially obtained LPS (Figure 1). LPS injections reduced sperm count on days 3, 7, and 42. Furthermore, sperm of grades A/B and D showed a significant decrease compared to the control group after day 7 (p=0.03).

Extracted lipopolysaccharide (LPS) from uropathogenic Escherichia coli compared to commercially available LPS. The arrow indicated the LPS band on the sodium dodecyl sulphate-polyacrylamide gel electrophoresis.
As shown in Table 1, the mean serum level of IL-17A was higher in the test group injected with LPS than in the control group on day 3, but this difference was not significant (p=0.8). Furthermore, other factors related to sperm quality and morphology did not show significant differences between the two groups, although a significantly lower sperm count was observed in the test group than in the control group (p=0.000). As presented in Table 2, which shows data from day 7, sperm grades A/B and D, sperm count, and sperm morphology variables were significantly lower in the test group than in the control group (p=0.03, p=0.03, p<0.001, and p<0.001, respectively).

Mean concentrations of IL-17A, sperm grades A/B, C, and D, sperm count, and abnormal sperm factors in the test and control groups on day 3

Mean concentrations of IL-17A, sperm grades A/B, C, and D, sperm count, and abnormal sperm factors in the test and control groups on day 7
The mean serum level of IL-17A in the group injected with LPS was higher than that of the control group on day 42, but this difference was not significant (p=0.1). However, sperm grades of A/B and D, sperm count, and sperm morphology variables were significantly lower in the group injected with LPS at day 42 than in the control group (p=0.005, p=0.004, p=0.000, and p=0.006, respectively) (Table 3). Table 4 presents a comparison of concentrations of IL-17A; sperm grades of A/B, C, and D; sperm count; and sperm morphology on days 3, 7, and 42. Sperm grade A/B and morphology variables significantly decreased over time (p<0.05).

Mean concentrations of IL-17A, sperm grades A/B, C, and D, sperm count, and abnormal sperm factors in the test and control groups on day 42

Mean concentrations of IL-17A, sperm grades A/B, C, and D, sperm count, and abnormal sperm factors in the test and control groups on days 3, 7, and 42
The microscopic studies of the tissues showed that the treatment of mice with 1 mg/kg/day of LPS did not give rise to a noticeable change in the morphology of the testis tissue on days 3 and 7, but significant changes in the cellular parenchyma and order were observed on day 42, including extensive necrosis of testicular parenchyma, severe karyolysis of parenchymal cells, extensive degradation of tissue parenchyma in seminiferous tubules, degradation of Sertoli cells, fragmentation of cell communication, destruction of the tissue organization, a lack of inflammatory cells, damage of the testicular network cells and the epididymis. No metaplastic changes were found in the seminiferous cells. In the inflammatory process, the factor of hyperemia was observed. Necrosis in Leydig cells and the interstitial cell tissue of the testes was also evident (Figure 2 and 3).

Optical micrograph of cell populations in seminiferous tubules stained with H&E at ×40 magnification. The control group with normal germinal epithelium (A). Histology of tubules after 3 (B), 7 (C), and 42 (D) days. The cells of the germinal epithelium were significantly reduced in group D.
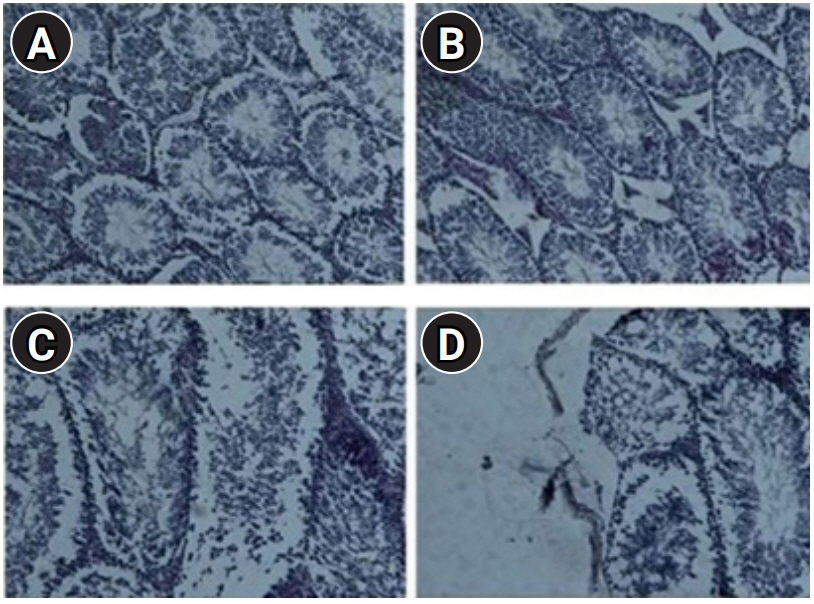
Optical micrograph, cross-section from testicular tissue stained with H&E at ×40 magnification. (A) The control group with normal cell populations. Histology of tubules after 3 (B), 7 (C), and 42 (D) days. It is clear that the lumen of tubules in lipopolysaccharide-treated mice showed extensive morphological changes, including loss of cell adhesions and necrosis after 42 days.
Discussion
Based on previous studies, bacterial infections and the subsequent immune response are one of the major causes of infertility in men [5]. Therefore, these infections at different stages of evolution, maturation, and transmission have adverse effects on sperm quality and thereby lead to reduced fertility or infertility. In order to explain the association of uropathogenic E. coli with fertility disorders caused by pathogens, the present study focused on the injection of LPS from this strain into BALB/c mice and evaluated its effects on serum and tissues. The results showed that injecting LPS of E. coli into healthy male mice during 42 days led to reductions in sperm count, poorer sperm quality, and defective sperm morphology compared to the control group. Seminiferous tubule damage was found, clearly due to the initial inflammation.
The findings of this study are consistent with those reported by Matsuura [21], who showed that in mice infected with Gram-negative bacteria, the presence of LPS stimulated the innate immune response and consequently the inflammatory response, both of which play vital roles in the elimination of pathogens. The common point in these studies was spermatogenic damage in response to Gram-negative bacteria, which account for a large proportion of genital infections. In the study of Bhatt et al. [22], 347 semen specimens were cultured and 62.9% of the isolates were Gram-negative. In the study of Sabra and Al-Harbi [23], more than 40% of sperm contamination was caused by Gram-negative bacteria. Pilatz et al. [24] found that E. coli was one of the most common symptomatic or asymptomatic infections of the urinary and genital tract and could change the sperm parameters such as motility. They also showed that the presence of E. coli caused the death of 80% of sperm in vitro [24]. Boguen et al. [25] reported that E. coli incubated with human sperm at a ratio of 1:2 gave rise to diminished sperm parameters, reduced motility, and increased levels of free reactive oxygen species. However, it should be noted that other bacteria such as Helicobacter pylori, as well as aerobic and anaerobic enteric bacteria, are directly and indirectly involved in male infertility [3].
The results showed that the injection of LPS led to changes in sperm parameters, such as a reduction in the sperm count, diminished motility, and unusual morphology of the sperm on days 7 and 42. Although some researchers believe that the presence of bacteria does not affect the morphology of spermatozoa [26,27], other studies have shown that the morphology is severely damaged. It has been pointed out that the immobility factor produced by E. coli has a major impact on the semen of men with oligospermia [28].
Previous research has demonstrated that LPS exerts direct or indirect effects on the seminiferous epithelium. Toll-like receptor 4 on the surface of leukocytes detects the LPS from Gram-negative bacteria, and subsequent signaling induces the production of proinflammatory cytokines such as IL-17A [29]. These cytokines can cause pathological damage in the cells and parenchyma of testicular tissue. In the present study, IL-17A levels on days 3, 7, and 42 after LPS injection increased and could have been correlated with sperm damage. This result is consistent with the study conducted by Babinets et al. [30], who showed that increased Il-17A levels were correlated with male infertility. A study on urinary tract infections (UTIs) caused by uropathogenic E. coli reported that IL-17 was a key mediator of the innate immune response to UTIs [13]. As a result, IL-17 could affect the sperm and testis tissue and may play a role in male infertility. Qian et al. [31] showed that high levels of IL-17 in seminal fluid could cause deficiencies in sperm motility, resulting in male infertility related to sperm motility. Huo et al. [32] also determined that extracellular fluoride could stimulate the immune response and cause high expression of the IL-17 signaling pathway in mice. Therefore, infection with Gram-negative bacteria including uropathogenic E. coli could damage sperm and testis tissue through both mechanisms: directly through immobilization of sperm and indirectly through soluble factors.
In conclusion, the results showed that LPS from uropathogenic E. coli could cause defects in sperm parameters, resulting in decreased motility, and could stimulate the immune system as an antigen. The production of proinflammatory cytokines such as IL-17 gives rise to an immune response in testicular tissue, which results in reduced count and function of sperm, as well as necrosis and damage to the testicular tissue. While mechanisms such as apoptosis have been identified as induction pathways of the death of germ cells, more studies are needed to obtain a clear understanding of the mechanisms involved in sperm immunopathology.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: KRK, MBK. Investigation: KRK,MBK, AS. Validation & Formal analysis: MS. Methodology: KRK, MBK, ART, AA. Project administration: MBK. Visualization: KRK, MBK, ART. Writing–original draft: KRK, MBK. Writing–review & editing: MBK, MS, AA, AS, FZ.
Acknowledgements
We appreciate the staff of the Laboratory of Microbiology, School of Paramedical and Medical Sciences, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
