The effects of sesame oil and different doses of estradiol on testicular structure, sperm parameters, and chromatin integrity in old mice
Article information
Abstract
Objective
Studies of the effects of estrogens on the male reproductive system have emphasized the role of these hormones in male fertility. Sesame oil has many phytoestrogenic compounds and may improve male fertility. This study investigated the effects of sesame oil and different concentrations of estrogen on sperm parameters and DNA integrity in male mice.
Methods
Twenty old NMRI (The Naval Medical Research Institute) male mice (40 weeks; weight, 30–35 g) were treated with sesame oil or different concentrations of estrogen (estradiol, 1 and 10 μL/kg/day) or received no treatment (controls). After 35 days, sperm parameters and DNA integrity were assessed and analyzed.
Results
Sperm count, progressive motility, and morphology were decreased in the group that received 10 μL/kg of estradiol. A remarkably lower percentage of DNA fragmentation and protamine deficiency were detected in the group that received 1 μL/kg of estradiol. In the groups that received sesame oil and 1 μL/kg of estradiol, the numbers of spermatogonia and Leydig cells were higher than in controls. The combination of sesame oil and 1 μL/kg of estradiol led to improved sperm parameters and chromatin and testicular structure.
Conclusion
Based on this study, consumption of sesame oil and a low concentration of estradiol may improve testicular function in older mice.
Introduction
Spermatogenesis is an essential process in the male reproductive system [1]. Associations of sex hormones with spermatogenesis, sperm survival, and DNA fragmentation have been demonstrated in previous research [2,3]. Sesame oil is an antioxidant agent containing large amounts of polyunsaturated fatty acids, lignin, and vitamin E. It has been proven that sesame oil can prevent DNA oxidative damage in an in vivo system [4]. The phytoestrogenic properties of sesame oil, similar to those of estradiol, may have additional effects on the improvement of sperm parameters and improve spermatogenesis through increasing epithelial proliferation and tubular thickening [5,6]. Aging has significant effects on sperm quality through the reduction of semen quality and an increase in DNA damage [7]. It has been observed that the increase in age has a detrimental effect on sperm DNA [8]. The production of reactive oxygen species (ROS) increases significantly in response to reduced production of steroidogenic enzymes. An increase in ROS also affects testicular morphology and reduces sperm parameters such as motility, concentration, and morphology [9,10]. These events may be associated with decreasing levels of estrogen and antioxidant agents associated with aging [11]. Estrogen affects the proliferation and arrangement of Sertoli cells, and it is also important for regulating the expression of the genes associated with cell adhesion [3,9,12]. The proteins that form adherent junctions include β-catenin and E-cadherin, which modulate intercellular junctions and stimulate hormones [13]. Furthermore, cadherins and catenins have effects on biological processes including intracellular messengers, signal transmission, and gene transcription. These actions may be associated with the regulatory function of steroids in reproductive tissues [14]. In addition, cadherins comprise a family of calcium-dependent glycoproteins that mediate cell-cell adhesion and sperm-oocyte interactions [15]. The presence of β-catenin is important for the adhesion of Sertoli cells, attachment to spermatids, and testicular-brain barrier function [16,17]. Many studies have been performed separately on the effect of sesame oil or estradiol on the testis; however, no study has yet compared the estrogenic effects of sesame or different concentrations of estradiol in old male mice. The aim of this study was to compare the estrogenic effects of sesame oil and different concentrations of estradiol on testicular structure, sperm parameters, chromatin/DNA integrity, and expression of E-cadherin and β-catenin genes in old mouse testis.
Methods
The study was approved by the Animal Ethics Committee of the Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran (IR.SSU.RSI. REC.1394.5). All protocols were performed according to the National Institute of Health Guidelines for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
1. Sample Collection
Twenty old NMRI (The Naval Medical Research Institute) male mice (mean age, 40 weeks; weight, 30–35 g) were purchased from the Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. All animals were kept in optimal housing and feeding conditions with a controlled temperature (22°C±2°C) and a 12-hour light/dark cycle. The mice were divided into four groups (n=5): the E2-1 group (1 μL/kg/day of estradiol was intraperitoneally injected for 35 days), the E2-10 group (10 μL/kg/day of estradiol was intraperitoneally injected for 35 days), the sesame oil group (10 μL/kg/day of sesame oil was intraperitoneally injected for 35 days), and the control group (no treatment was done).
After 35 days (one cycle of spermatogenesis in mice), animals were sacrificed by cervical dislocation. The left cauda epididymis was removed and cut with a pair of syringes to transferred into Ham's F10 medium for the analysis of sperm parameters and DNA integrity. The left testicular tissue samples were used for the analysis of E-cadherin and β-catenin expression by molecular assays. To evaluate histological changes, the right testes were fixed in 4% paraformaldehyde solution.
2. Sperm Parameters
After 30 minutes of incubation, sperm count, motility, and morphology were analyzed. A Makler chamber was used for the sperm count. Sperm motility was categorized as progressive, nonprogressive, and immotile spermatozoa. The percentage of sperm cells with normal morphology in the head, neck/mid-piece, and tail were obtained by Diff-Quik staining using light microscopy (×1,000 magnification) (Figure 1A) [18].
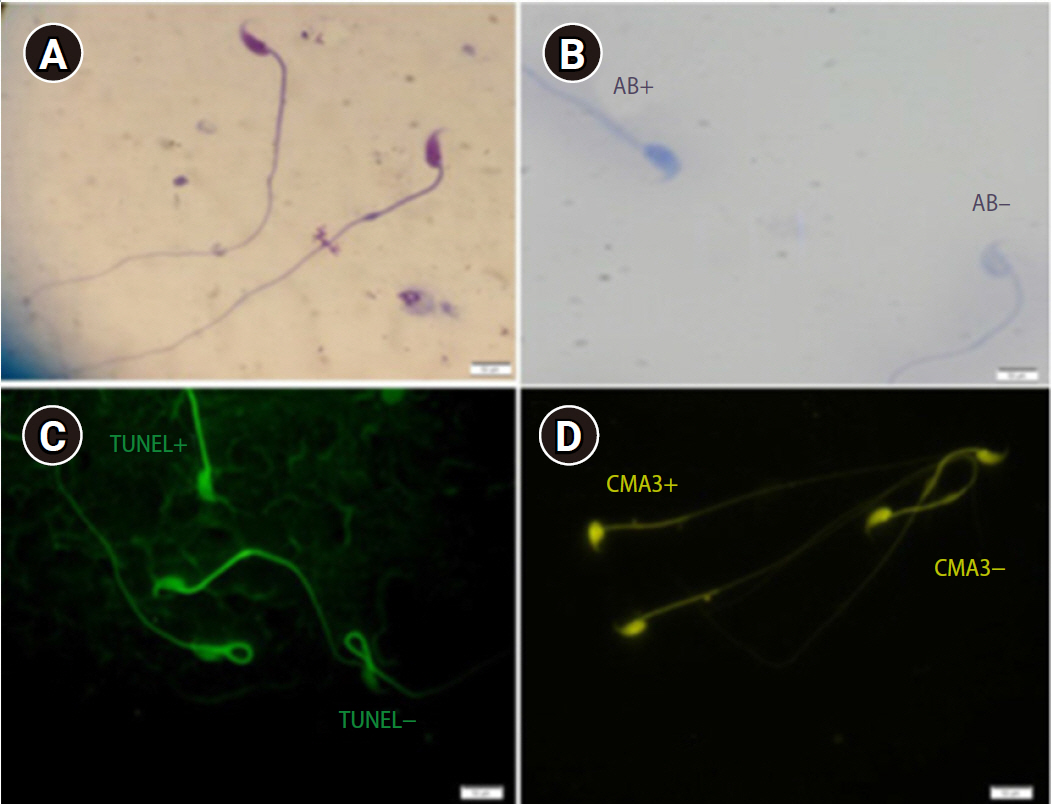
(A) Sperm morphology determined using Diff-Quik staining (×1,000). (B) AB staining assessing sperm chromatin status. Sperm heads with immature nuclear chromatin were shown as dark blue (AB+) and those with mature nuclei (AB–) were detected as light blue (×1,000) (C) TUNEL assay: apoptosis-positive cells are brilliant fluorescent green (TUNEL+) and apoptosis-negative cells are pale and opaque green (TUNEL–) (×1,000). (D) CMA3-positive cells (CMA3+) were seen as bright yellow, whereas cells with no protamine defects stained dark yellow (CMA3–) (×1,000 magnification). AB, aniline blue; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; CMA3, chromomycin A3.
3. Sperm Chromatin Integrity
Aniline blue (AB) staining was applied to evaluate sperm chromatin integrity based on the residual histones in the chromatin structure. Briefly, slides were prepared by smearing, air-drying, and fixing a sperm sample. Then, the sample was incubated for 30 minutes in 3% glutaraldehyde in phosphate-buffered saline (PBS) at room temperature. The smears were stained in 5% aqueous AB solution (pH 3.5) for 10 minutes. Afterward, the slides were rinsed and evaluated at ×1.000 magnification. Immature and/or abnormal spermatozoa with additional histones were seen in dark blue and mature nuclei were detected as light blue (Figure 1B) [19].
4. Sperm DNA Fragmentation
The percentage of sperm apoptosis was determined by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using a commercially available kit (In Situ Cell Death Detection Kit, fluorescein, Roche, USA). Spermatozoa with normal DNA show the background fluorescent color, while sperm with high DNA fragmentation has many 3-OH ends, resulting in a strong fluorescent color. Firstly, the smears were fixed in methanol solution for 4 minutes. The slides were washed with PBS for 5 minutes three times. Later, they were incubated with blocking solution for 15–20 minutes at 15°C–25°C in a dark room. Samples were incubated with 0.1% (v/v) Triton X-100 containing 0.1% (w/v) sodium citrate for 10 minutes on ice. Slides were again washed three times with PBS for 5 minutes and were stained with 50 μL of TUNEL reaction mixture for 1 hour at 37°C in a dark and humidified atmosphere. Then, they were examined under a fluorescent microscope at ×1,000 magnification (BX51; Olympus, Tokyo, Japan) (Figure 1C) [20].
5. Sperm Protamine Deficiency
Protamine deficiency in sperm was analyzed by chromomycin A3 (CMA3), which is bright yellow. The smears were fixed immediately with Carnoy solution for 10 minutes at 4°C. Each slide was treated with 100 μL of CMA3 solution for 10 minutes in a dark room (Sigma-Aldrich, St. Louis, MO, USA). The slides were rinsed in McIrvin buffer and air-dried. The slides were analyzed using fluorescent microscopy with suitable filters (×400 magnification) (Figure 1D) [20].
6. Gene Expression
The testis tissue of each mouse was used for RNA extraction. Total RNA was extracted by the QuantiTect, RNeasy Micro kit (Qiagen, Hilden, Germany), following a slight modification of the manufacturer’s protocol in a total volume of 14 μL. Concentrations of extracted RNA were measured by a Nanodrop spectrophotometer (Thermo Scientific, Waltham, MA, USA). Subsequently, 1,000 ng/μL of extracted total RNA was reverse-transcribed using the Revert Aid First Strand cDNA synthesis kit (Thermo Fisher Scientific Inc., Waltham, MA, USA) according to the manufacturer’s instructions. For negative control samples, the reverse transcriptase enzyme or the RNA template was removed from the reactions. Synthetic cDNA was stored at –80°C until quantitative real-time polymerase chain reaction was performed to assess the relative gene expression levels of the genes encoding E-cadherin and β-catenin in testis tissue from all groups, and the β-actin gene was considered as a reference gene for normalization. Relative expression of the genes was calculated using the QuantiTect SYBER Green RT-PCR kit (Applied Biosystems, Foster City, CA, USA) by an RT-PCR thermocycler (ABI 7500 Step One, Applied Biosystems). Primer sequences for genes are listed in Table 1. Amplification of all runs was performed in duplicate by an expert laboratory assistant blinded to the study design.
7. Testicular Histology
Testicular sections were prepared with a 5-μm thickness. Furthermore, hematoxylin and eosin staining was performed to analyze the diameter of seminiferous tubules using an optical microscope (BX51, Olympus). In each section, three fields and at least 20 tubules were randomly selected. In this regard, large and small diameters were measured in each tubule and the average diameter was recorded. The thickness of the germinal epithelium layer was also calculated by subtracting the inner diameter of the tubule from the overall diameter of the seminiferous tubule [21]. Sertoli cells, spermatogonia, primary spermatocytes, spermatids, and Leydig cells were counted in each testis.
8. Statistical Analysis
One-way analysis of variance was used to compare the data and Pearson correlation coefficients were used to quantify the relationships between the variables, with p-values <0.05 considered to indicate statistical significance. The data were analyzed using IBM SPSS ver. 20 (IBM Corp., Armonk, NY, USA) and GraphPad software (GraphPad Inc., La Jolla, CA, USA) was used to draw the charts.
Results
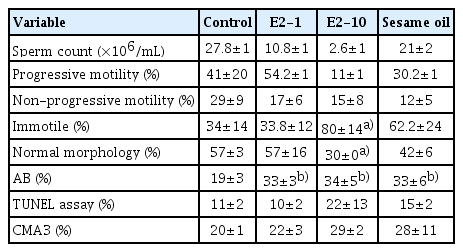
As shown in Table 2, the percentage of progressive motility was significantly lower in the E2-10 group than in the control group (p<0.05). There was no significant difference in the proportion of nonprogressive sperm across all groups. The percentage of immotile sperm was higher in the E2-10 and sesame oil groups than in the control group (p<0.05). Normal morphology (Figure 1A) was significantly lower in the E2-10 group than in the control group (p<0.05) (Table 2). The rate of abnormal chromatin in AB staining in the E2-10 and sesame oil groups was higher than in the control group and the low-dose estradiol group (p<0.001) (Table 2, Figure 1B). As shown in Table 2, a difference was observed between the E2-10 and control groups in the TUNEL assay (p<0.05) (Figure 1C). The results of CMA3 staining showed higher percentages of abnormal sperm in the E2-10 and sesame oil groups than in the control group (p<0.001) (Table 2, Figure 1D). The expression of β-catenin and E-catenin was quantified in all groups (Table 1). The relative expression of β-catenin mRNA in the E2-1 (p=0.002) and E2-10 (p=0.012) groups was higher than in the control group (Figure 2A), but the relative expression of E-catenin mRNA did not significantly differ across all groups (Figure 2B). The number of spermatogonia showed no significant difference in any group compared to the control group (Figure 3A). The E2-10 group displayed higher primary spermatocyte, spermatid cell, and Sertoli cell counts compared to the controls at 35 days (p<0.05) (Figure 3B-D). The Leydig cell count was significantly higher in the E2-1 and E2-10 groups (p<0.001) (Figure 3E). In the E2-10 group (Figure 4A), the lumen diameter was significantly lower than that of the control animals (p<0.05), and the cellular diameters of the seminiferous tubules were also significantly higher in the E2-1 (p<0.05) and E2-10 (p<0.05) groups (Figure 4B). The total diameter did not significantly vary across all groups (Figure 4C).
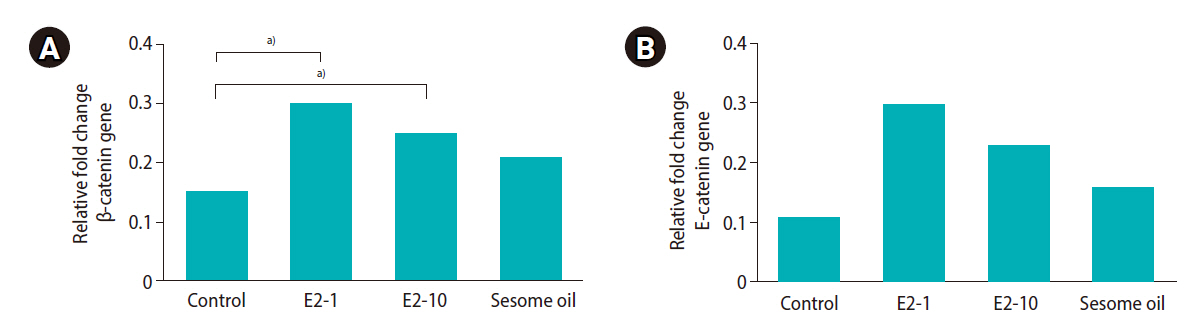
(A) Evaluation of mRNA levels of the β-catenin gene. The E2-1, E2-10 and sesame oil group were intraperitoneally injected with 1 μL/kg/day of estradiol, 10 μL/kg/day of estradiol, and 10 μL/kg/day of sesame oil for 35 days, respectively. (B) Evaluation of mRNA levels of the E-cadherin gene. a)Significant mRNA levels of β-catenin and E-cadherin (p≤0.05) when compared to the control group.
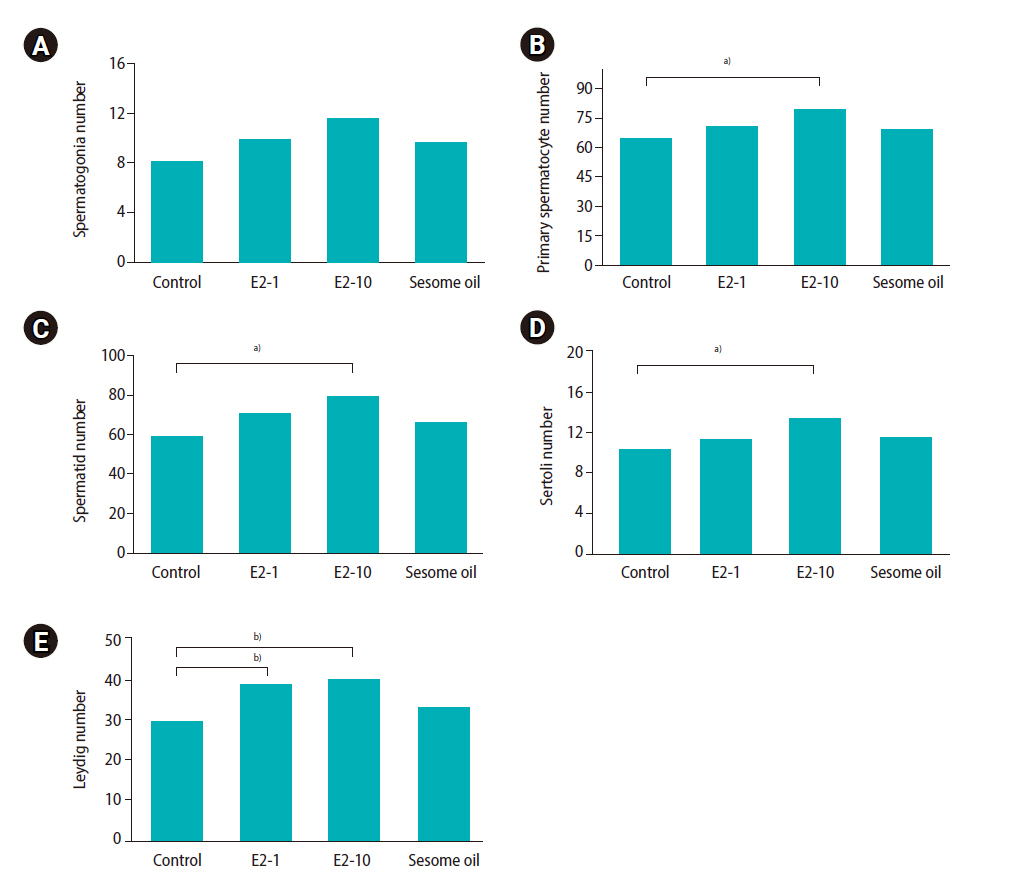
Mean and standard error of cell counts in seminiferous tubules in the E2-1, E2-10, and sesame oil groups, which were intraperitoneally injected with 1 μL/kg/day of estradiol, 10 μL/kg/day of estradiol, and 10 μL/kg/day of sesame oil for 35 days, respectively. (A) Spermatogonia cell count, (B) primary spermatocyte cell count, (C) spermatid cell count, (D) Sertoli cell count, (E) Leydig cell count. Significant differences between groups: a)p<0.05, b)p<0.001.
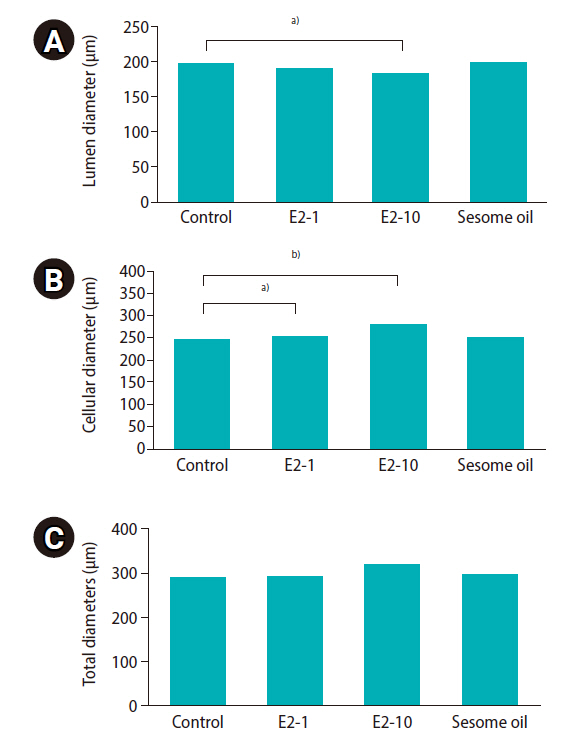
Mean and standard error of stereological indices of seminiferous tubules in the E2-1, E2-10, and sesame oil groups, which were intraperitoneally injected with 1 μL/kg/day of estradiol, 10 μL/kg/day of estradiol, and 10 μL/kg/day of sesame oil for 35 days, respectively. (A) Lumen diameter (μm), (B) cellular diameter (μm), (C) total diameters (μm) of the tubule. Significant differences between groups: a)p<0.05, b)p<0.001.
Discussion
Spermatogenesis decreases daily with a rate of 30% in men above 50 years old [22-24], and aging affects sperm parameters such as sperm motility and viability, as well as sperm chromatin status. Sesame oil is important source of phytoestrogens and has estrogenic properties [25]; furthermore, it can improve sperm count and motility. Thus, it has been suggested that sesame oil could be considered as an effective agent for improving the condition of epididymal spermatozoa [26]. An appropriate dose of sesame oil for its high antioxidant activity may have effective anti-aging results due to its ability to neutralize physiological ROS. Increased ROS levels in semen cause sperm dysfunction and DNA damage by oxidative stress, accounting for 25% of cases of induced male infertility [27,28]. This study showed that sesame oil was more effective than estradiol at 10 μL/kg in improving sperm parameters such as progressive motility and morphology. The more favorable effects of sesame oil may be due to its antioxidant properties and ability to bind to antioxidant enzymes within the cell [29]. In contrast, Abbasi et al. [30] reported that sesame oil could improve sperm parameters in diabetic rats, potentially due to hormonal imbalance and the presence of abundant ROS in those animals. Lubbert et al. [31] reported that a high dose (60 μg/day) of estrogen and a long duration of treatment could impact sperm motility and count [9]. Our results demonstrated the effects of an injection of 1 μL/kg/day of estradiol on sperm motility. In addition, we observed that a higher concentration of estradiol (10 μL/kg/day of estradiol) had a negative effect on sperm motility, but did increase the sperm count. Lubbert et al. [31] found that although a high dose of estrogen (60 μg/day) reduced the sperm count, low doses (20 μg/day) did not have a negative effect on the sperm count in adult men. The effect of a high dose of estrogen on the sperm count was observed a few days after injection. Steroid hormones play vitally important roles in the maintenance of male reproductive function [32]. and sperm DNA fragmentation and progressive motility are important factors for the evaluation of fertility [33-35]. Based on our data, a low dose of estradiol may lead to improvement in sperm parameters, especially chromatin quality and DNA fragmentation, rather than a high dose of estradiol. In this study, CMA3, TUNEL, and AB assays were used to evaluate chromatin and DNA status. The results of CMA3, TUNEL, and AB tests showed that low concentrations of estradiol and sesame oil were more appropriate than higher concentrations of estradiol. In another study, Ebrahimi et al. detected that sesame oil had anti-apoptotic effects on sperm [36]. Our data showed that 1 μL/kg/day of estradiol led to the most favorable results in the TUNEL assay. The CMA3 assay is a suitable method for detecting protamine deficiency in sperm chromatin [37-40]. Although a few studies have been conducted on the effect of estrogen on the gene expression of Sertoli-spermatid binding proteins [17], to the best of our knowledge, no study has reported the effects of different exogenous estrogen doses on the expression of E-cadherin and β-catenin. Our study showed that a low concentration of estradiol downregulated the β-catenin gene, which plays a role in germ cell-to-Sertoli cell attachment and mediates proteins in cellular connections, but there was no effect on the expression of the gene coding for E-cadherin, which also plays an important structural role [41]. A higher dose of estradiol (10 μL/kg/day) improved spermatogenesis in mice. This finding was confirmed by a significantly higher number of spermatogonia cells in the animals that received 10 μL/kg/day of estradiol than was found in the control group. Furthermore, the numbers of primary spermatocytes, spermatids, Sertoli cells, and Leydig cells were significantly higher in the E2-10 group than in the control group. Toyama et al. [42] studied six different doses of estradiol and they reported that the effects of estradiol on the male reproductive system were dose-dependent [43]. It has also been found that estradiol synthesis by seminiferous cells plays an important role in tubal hormonal regulation and spermatogenesis improvement. and estradiol and platelet-derived growth factor likely induce proliferation of seminiferous cells in both a dose-dependent and dose-insensitive manner [44]. Moreover, incubation of the seminiferous tubules with estradiol inhibits apoptosis and produces germ cells in these tubules. Therefore, estradiol is a very important hormone for the survival of germ cells [45]. In addition, the estradiol beta receptor is present in Sertoli cells and estradiol exerts its effects through this receptor [46]. Our results showed an a higher cellular diameter of seminiferous tubules in mice that received 10 μL/kg/day of estradiol than was found in the control group. Moreover, MacCalman et al. [47] showed that estradiol increased the stimulating effects of follicle-stimulating hormone (FSH) and N-cadherin (a protein that is essential for adhesion and internal cell adhesion in the seminal epithelium) mRNA levels. The interaction between FSH and estradiol in Sertoli cells stimulates the mitotic activity of these cells [48-50]. Therefore, the increase of the cellular diameter was probably due to the interaction of injected estradiol with FSH. Furthermore, changes in tubal diameter could occur due to an increase in the number of germ lineage cells within the seminiferous tubules [51]. According to Shittu et al. [52], sesame oil raises testosterone levels and testosterone increases spermatogenesis in male animals. The aqueous extract of sesame leaves has an antioxidant effect and significantly increases the number of spermatogonia, seminiferous tubules, and testosterone levels. According to other researchers, sesame phytoestrogens bind to testicular estrogen receptors and stimulate spermatogenesis through the proliferation of epithelial cells and sex cells [52,53]. In the sesame oil and high-dose estradiol groups, a higher rate of chromatin and DNA damage was observed than in the control group and the low-dose estradiol group. Low doses of estradiol had a greater effect on sperm motility, in addition to exerting less chromatin and DNA damage. Therefore, it is recommended to use a combination of a low dose of estradiol and sesame oil. This combination may lead to a reduction of age-related ROS by balancing the oxidants and antioxidants in the cell. These are novel findings.
Despite the beneficial effects of high-dose estradiol on testicular function, we recommend that low doses of estradiol or sesame oil may play an important role on optimizing sperm parameters and chromatin quality in older mice.
Acknowledgements
The authors would like to thank Yazd Reproductive Science Institute for financial support of the current study.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: ART, HZZ. Funding acquisition: MM, MP. Methodology: MM, ART. Project administration: AN, SGE. Writing–original draft: ART. Writing–review & editing: AK.