Effect of evaporation-induced osmotic changes in culture media in a dry-type incubator on clinical outcomes in in vitro fertilization-embryo transfer cycles
Article information
Abstract
Objective
This study investigated whether adding outer-well medium to inhibit osmotic changes in culture media in a dry-type incubator improved the clinical outcomes of in vitro fertilization-embryo transfer (IVF-ET) cycles.
Methods
In culture dishes, the osmotic changes in media (20 µL)-covered oil with or without outer-well medium (humid or dry culture conditions, respectively) were compared after 3 days of incubation in a dry-type incubator. One-step (Origio) and G1/G2 (Vitrolife) media were used.
Results
The osmotic changes in the dry culture condition (308 mOsm) were higher than in the humid culture conditions (285–290 mOsm) after 3 days of incubation. In day 3 IVF-ET cycles, although the pregnancy rate did not significantly differ between the dry (46.2%) and humid culture (51.0%) groups, the rates of abortion and ongoing pregnancy were significantly better in the humid culture group (1.5% and 49.5%, respectively) than in the dry culture group (8.3% and 37.8%, respectively, p<0.05). In day 5 IVF-ET cycles, the abortion rate was significantly lower in the humid culture group (2.2%) than in the dry culture group (25.0%, p<0.01), but no statistically significant difference was observed in the rates of clinical and ongoing pregnancy between the dry (50.0% and 25.0%, respectively) and humid culture groups (59.5% and 57.3%, respectively) because of the small number of cycles.
Conclusion
Hyperosmotic changes in media occurred in a dry-type incubator by evaporation, although the medium was covered with oil. These osmotic changes were efficiently inhibited by supplementation of outer-well medium, which resulted in improved pregnancy outcomes.
Introduction
Even brief exposure of preimplantation mouse embryos to high-osmolality culture medium (>300 mOsm/kg) in the absence of osmolytes resulted in impaired development [1-3]. The detrimental effect of hyperosmolality has also been reported in the development of various mammalian embryos, including murine [4-6], rat [7], porcine [8,9], and bovine [10,11] embryos. Moreover, in vitro two-cell blocks of mouse embryos were significantly alleviated by culturing them in low-osmolarity (250 mOsm) medium compared to high-osmolality (>300 mOsm) medium [12]. Biggers et al. [3] reported that when the NaCl concentration was increased in the medium, the intracellular Na+/K+ ratio dramatically increased, which was detrimental to mouse embryo development. The detrimental effects of hyperosmolality occur by triggering cell shrinkage, oxidative stress, protein carbonylation, mitochondrial depolarization, DNA damage, cell cycle arrest, and apoptosis [13,14].
From a different point of view, an increase in extracellular osmolality can promote water flux out of the cell, triggering cell shrinkage and intracellular dehydration [15]. Intracellular water loss interferes with many cellular functions, including DNA synthesis and repair, transcription, protein translation and degradation, and mitochondrial function. As a result, cell cycle progression and cell proliferation are arrested [13]. Dry-type incubators are now widely used instead of humid-type incubators due to the development of infrared CO2 sensors [16] and the low possibility of microorganism overgrowth [17]. In addition, dry-type incubators have smaller culture chambers than humid-type incubators, is advantageous in terms of the short recovery time for gas and temperature after the door is opened. However, concerns about the possible change in the osmolality of the medium by evaporation remain a reason why clinicians may hesitate to use a dry-type incubator. Although overlying culture dishes with oil could inhibit the shift in the osmolality of the medium, whether it completely eliminates the change in osmolality remains controversial [18]. Recently, human embryos cultured in dry-type incubators showed significantly lower implantation and clinical pregnancy rates than those cultured in humid-type incubators [18].
The present study was performed to compare osmotic changes in culture media covered in oil in various types of culture dishes, and to investigate whether a beneficial effect on clinical outcomes in in vitro fertilization-embryo transfer (IVF-ET) cycles could be obtained by supplementation with outer-well medium to inhibit osmotic changes in culture media in a dry-type incubator.
Methods
This retrospective study was approved by the Institutional Review Board of Mamapapa and Baby Clinic (IRB No. 2019-10-01), and was conducted from August 2018 to August 2019.
1. Patients
In total, 796 IVF-ET cycles in 673 patients were analyzed in the present study. Twelve patients who underwent their first 3-day IVF-ET cycles using a cell culture dish, but failed to show implantation or ongoing pregnancy, completed their second IVF-ET cycles using a GPS dish to compare the clinical outcomes between cell culture and GPS dish cycles. After a comparison of these 12 patients, we changed the culture medium from 1-Step medium to G1/G2 medium; the remaining 772 IVF-ET cycles in 661 patients were performed using G1/G2 medium. Of these cycles, 168 IVF-ET cycles (156 day 3 IVF-ET cycles + 12 day 5 IVF-ET cycles) in 159 patients used cell culture dishes, while 628 IVF-ET cycles (539 day 3 IVF-ET cycles + 89 day 5 IVF-ET cycles) in 514 patients used GPS dishes. In the early stage of the study, we found that using the GPS dish had a beneficial effect on clinical outcomes, and after confirming a significant improvement in pregnancy outcomes in the GPS dish group, we completely changed to GPS dishes. The ultimate goal of our studies is to improve the pregnancy rate in IVF cycles; therefore, we could no longer use the cell culture dishes. This change resulted in the difference of the number of cycles between the cell culture and GPS dish groups.
2. Ovarian stimulation and oocyte aspiration
Controlled stimulation for IVF cycles was performed with a mild stimulation protocol using a combination of a gonadotropin-releasing hormone (GnRH) antagonist and gonadotropins. Patients received 150 IU of recombinant follicle-stimulating hormone (Gonal-F; Merck Serono, Darmstadt, Germany) alone as a daily injection from cycle day 3 until the day when human chorionic gonadotropin (hCG) was administered. The GnRH antagonist (Cetrotide, Merck Serono) was initiated on the day when the leading follicle reached a diameter of 14 mm. Ovarian follicular development was monitored by transvaginal ultrasonography. When the leading follicles reached ≥18 mm in maximum diameter, as detected by sonography, ovulation was induced by injecting 250 µg of hCG (Ovidrel, Merck Serono). Oocyte retrieval was performed using 20-gauge ovum aspiration needles (Cook Medical, Bloomington, IN, USA) under standard transvaginal ultrasound guidance 35–36 hours after hCG administration. The luteal phase was supported by progesterone injection or vaginal gel (Crinone, Merck Serono). A serum β-hCG test was performed about 2 weeks after oocyte retrieval. Clinical pregnancy was confirmed by the visualization of a gestational sac. Ongoing pregnancy was defined as a pregnancy that was maintained for over 20 weeks of gestation.
3. Embryo culture in vitro
1-Step (Origio, Malov, Denmark) and G1/G2 media (Vitrolife, Göteborg, Sweden) were employed in IVF-ET cycles. The culture dishes for IVF and embryo culture were prepared and incubated in a humid-type incubator (HERAcell 150i; Thermo Scientific, Waltham, MA, USA) overnight to achieve an optimal pH of 7.2–7.3. Fertilized oocytes were individually cultured in 20-μL drops of the culture medium covered in oil for 3–5 days until transfer, in 6.0% CO2, 5% O2 and 89.0% N2, in a dry-type incubator (Miri; ESCO, New Haven, CT, USA). The embryos were cultured in a cell culture dish without outer-well medium (dry culture condition) or in a µ-drop GPS dish with outer-well medium (humid culture condition). In day 5 IVF-ET cycles of the cell culture dish and GPS dish groups, dish change was performed on day 3 by transferring the embryos to a new culture dish prepared on day 2, to inhibit osmotic changes induced by evaporation and to serve as a new culture medium.
4. Estimation of osmolality changes in media
Osmotic changes in micro-drops (20 µL) of medium covered in oil and with or without outer-well medium supplementation were compared in the following types of culture dishes: cell culture (Corning Inc., Corning, NY, USA), µ-droplet culture (Vitrolife), microwell culture (DNP, Kashiwa, Japan), and µ-drop GPS (LifeGlobal, Brussels, Belgium) (Figure 1). Ham’s F-10 (Gibco, Grand Island, NY, USA) and G1 (Vitrolife) were used to investigate changes in osmolality. The basic osmolality of the media was about 280 and 275 mOsm/kg, respectively. The osmolality of media was estimated with a micro-osmometer (Advanced, Norwood, MA, USA), after 3 days of incubation in the dry-type incubator.
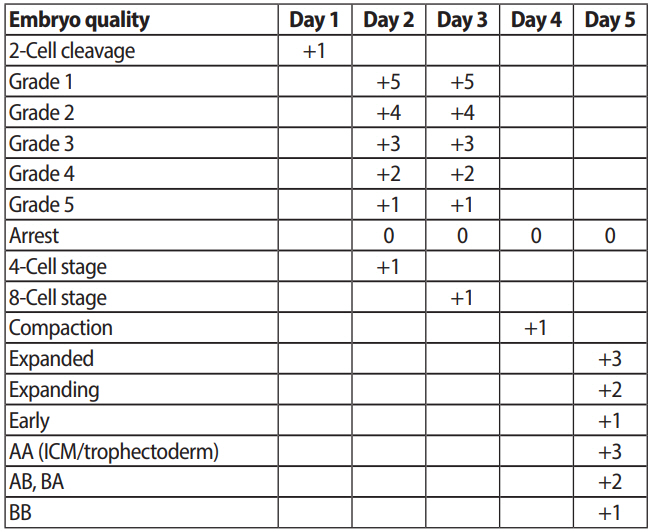
5. Embryo sequential scoring
The quality of embryos was daily evaluated and scored according to the developmental stage and speed, as well as the shape of blastomeres and degree of fragmentation (Figure 2). The embryo grades and scores were as follows: grade 1 (+5 points), no fragmentation with equal-sized blastomeres; grade 2 (+4 points), <10% fragmentation with equal-sized blastomeres or no fragmentation with unequal-sized blastomeres; grade 3 (+3 points), 10%≤ fragmentation <25%; grade 4 (+2 points), 25%≤ fragmentation <50%; grade 5 (+1 point), ≥50% fragmentation; arrested embryos (0 point). When an embryo showed a normal developmental speed and stage, an additional point was given (+1 point), such as two-cell cleavage on day 1, four-cell stage on day 2, eight-cell stage on day 3, or compaction on day 4. It has been reported that fast cleavage speed on day 4 showed an association with high aneuploidy and low blastocyst formation rates. Moreover, in our accumulated data, the pregnancy rate of embryos with a normal cleavage speed was higher than high-speed embryos or low-speed embryos. The quality of blastocysts were evaluated separately in terms of the inner cell mass and trophectoderm; each cell type was classified as grade A to C, and points were given according to the grades, as follows; A (+3 points), B (+2 points) and C (+1 point). The embryos with the highest cumulative scores were selected for transfer.
6. Statistical analysis
Statistical analysis was performed with SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA). Means and standard deviations were calculated for all variables. The Student t-test was employed to analyze differences in patients’ age, endometrial thickness, number of oocytes retrieved, scores of embryos, and number of embryos transferred between the dry and humid culture groups. Differences in clinical outcomes between the two groups were analyzed by the chi-square test, and p-values <0.05 were considered to indicate statistical significance.
Results
1. Osmotic changes in media according to the type of culture dish after 3 days of incubation in a dry-type incubator
Osmotic changes in 20-µL droplets of Ham’s F-10 (280 mOsm) and G1 (275 mOsm) media according to the various types of culture dishes were compared after 3 days of incubation in the dry-type incubator (Table 1). The osmolality of Ham’s F-10 in the cell culture dishes (301.1 mOsm) in the dry-type incubator was higher than that (286.1 mOsm) in the humid-type incubator, although the dishes were covered in 6 mL of oil. Compared to the cell culture dishes with dry culture conditions (without outer-well medium, 301.1 mOsm), the humid culture conditions (with outer-well medium) using the µ-droplet culture (290.4 mOsm), Microwell culture (285.3 mOsm) and µ-drop GPS (287.2 mOsm) dishes showed lower osmolality. The osmolality of the G1 medium in the cell culture dishes (293.7 mOsm) and µ-drop GPS dishes without outer-well medium (293.6 mOsm) was higher than that in the µ-drop GPS dishes with outer-well medium (285.0 mOsm) after 3 days of incubation in the dry-type incubator. Although there was no difference in the osmotic change between the GPS dish and the other dishes supplemented with outer well medium, we selected the µ-drop GPS dishes to use for human embryo culture because of their suitability for our culture system.
2. Clinical outcomes of the first (cell culture dish, dry culture) and second day 3 IVF-ET cycles (GPS dish, humid culture) in the same 12 patients using 1-Step (Origio) medium
As shown in Table 1, supplementation of outer-well medium (humid culture condition) was efficient for maintaining the osmolality of media in the dry-type incubator. To investigate the possible beneficial effects of the humid culture condition, in the same 12 patients, the first IVF-ET cycle was performed using the cell culture dishes and the second IVF-ET cycle was performed using the GPS dishes (Table 2).

Clinical outcomes of the 1st (cell culture dish, Dry culture) and 2nd Day 3 IVF-ET cycles (GPS dish, Humid culture) in the same 12 patients using 1-step (Origio) medium
There were no significant differences in the characteristics of the same 12 patients between the first and second IVF-ET cycles, because the second IVF-ET cycles were performed within 6 months after the completion of the first IVF cycles. Although the rates of clinical pregnancy and abortion (50.0% and 0%) in the second IVF-ET cycles were more favorable than those (30.7% and 30.7%, respectively) in the first IVF-ET cycles, the difference was not statistically significant. However, the ongoing pregnancy rate (50.0%) of the second IVF-ET cycles was significantly higher than the rate (0%, p<0.05) of the first IVF-ET cycles. When the two IVF cycle groups were subdivided into intracytoplasmic sperm injection (ICSI) and conventional IVF cycle groups, no differences were found in the characteristics of patients and clinical outcomes between the subgroups of the first and second IVF-ET cycle groups.
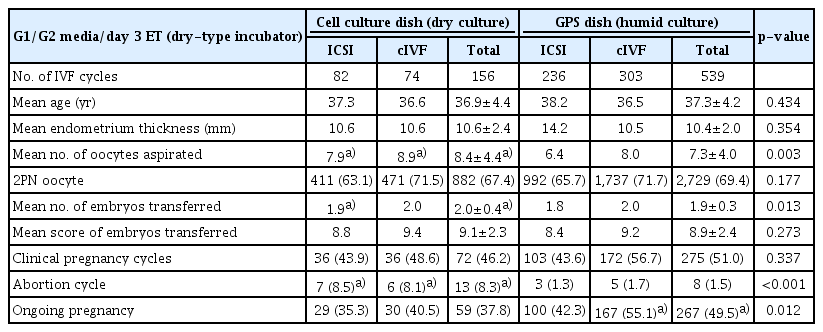
3. Clinical outcomes of day 3 IVF-ET cycles in the cell culture dish (dry culture) and GPS dish (humid culture) groups using G1/G2 medium (Vitrolife)
In the day 3 IVF-ET cycles, there were no significant differences in the mean age (36.9±4.4 and 37.3±4.2), endometrial thickness (10.6±2.4 and 10.4±2.0 mm), score of transferred embryos (9.1±2.3 and 8.9±2.4) and fertilization rate (67.4 and 69.4%) between the dry (156 cycles) and humid culture (539 cycles) groups (Table 3). However, the numbers of oocytes retrieved (8.4±4.4) and transferred embryos (2.0±0.4) in the dry culture group were significantly higher than those in the humid culture group (7.3±4.0 and 1.9±0.3, respectively, p<0.05). Nevertheless, the rates of abortion and ongoing pregnancy in the humid culture group (1.5% and 49.5%) were significantly more favorable than those in the dry culture group (8.3% and 37.8%, respectively, p<0.01), although there was no significant difference in the clinical pregnancy rate between the dry (46.2%) and humid culture (51.0%) groups. When the two culture groups were subdivided into ICSI and conventional IVF cycle groups, the characteristics of patients and clinical outcomes of the subgroups showed a similar pattern to the total IVF cycles between the two culture groups.
4. Clinical outcomes of day 5 IVF-ET cycles in the cell culture dish (dry culture) and GPS dish (humid culture) groups using G1/G2 medium
In the day 5 IVF-ET cycles, there were also no differences in the mean age (36.1±3.9 and 36.3±3.4 years, respectively), endometrial thickness (10.8±1.7 and 10.6±2.1 mm, respectively), the number (1.0±0.0 and 1.0±0.2, respectively) and score (16.5±1.8 and 15.3±3.0, respectively) of embryos transferred, or the fertilization rate (77.7% and 76.2%, respectively) between the dry (12 cycles) and humid culture (89 cycles) groups (Table 4). However, the number of oocytes aspirated (12.3±4.7) in the dry culture group was significantly higher than that in the humid culture group (9.9±3.4, p<0.05). The abortion rate in the humid culture group (2.2%) was significantly lower than that in the dry culture group (25.0%, p<0.01), but no statistically significant difference was observed in the rates of clinical and ongoing pregnancy between the dry (50% and 25.0%) and humid culture groups (59.5% and 57.3%, respectively), most likely due to the small number of cycles in the dry culture group. When the day 5 IVF-ET cycles in the two culture groups were also subdivided into ICSI and conventional IVF cycle groups, the characteristics of patients and clinical outcomes of the subgroups showed a similar trend to the total IVF cycles between the two culture groups.
Discussion
When the osmolality of the medium increases above a certain threshold, embryo development is compromised [1,5] and apoptosis is increased [19]. Hyperosmolality (>300 mOsm) was shown to have a detrimental effect on preimplantation mouse embryo development in previous studies [4-6]. However, paradoxically, the osmolality of oviductal fluid is about 340 mOsm [2,20], which is high enough to impair mouse embryo development in vitro. However, unlike in vitro, mouse zygotes have no difficulty developing in vivo, which suggests that an unknown mechanism may exist that helps them overcome the detrimental effect of hyperosmolality in vivo [1].
One possible mechanism is the use of various amino acids as organic osmolytes by embryos. The addition of various organic osmolytes, including taurine [21-24], hypotaurine [25], and glutamine [26], were beneficial for embryo culture. Glycine has also been shown to protect mouse [3] and rabbit embryos [27] against the effects of high NaCl levels. Indeed, over 70% of zygotes developed to the blastocyst stage when 1 mM glutamine was present at 310 mOsm, but only about 10% did so in the absence of glutamine [1]. At 310 mOsm of KSOM medium in the absence of glycine, over 80% of outbred mouse zygotes were arrested at the two-cell stage. However, in the presence of glycine, 60% of the zygotes developed to the blastocyst stage [12]. Furthermore, more blastocysts formed when bovine zygotes were cultured in a 247 mOsm medium (34.6%) than in a 286 mOsm medium (17.0%) in the presence of 1 mM glycine [28]. The beneficial effect of organic osmolytes can be explained by previous findings that amino acids, including osmolytes, are present in oviductal and uterine fluid [29-31] and carry out various physiological functions in the preimplantation embryo, including ATP production [32], ammonium detoxification [33,34] and maintaining the redox balance [35].
Baltz and Tartia [36] suggested a possible answer for the question “Why did lower osmolarity support embryo development in culture?” They proposed that the osmolality in the in vivo environment of early preimplantation embryos may be lower than in blood plasma (280 mOsm). This possibility conflicts with previous reports that the osmolality of oviductal fluid is about 340 mOsm [2,20]. Although the oviduct is the major in vivo environment for early-stage embryos, the follicle can be seen as the birthplace of the oocyte and embryo. In mice, a large amount of follicular fluid moves into the ampulla of the oviduct with all ovulated oocytes via the ovarian bursa. In human, immediately after ovulation, a small amount of follicular fluid flows weakly from the ovarian wall to the ampulla of the oviduct to help the sliding movement of the oocyte. This means that follicular fluid may play an important role in determining the oviductal environment for early-stage embryos. In our preliminary test (data not published), we compared the osmolality of human follicular fluid and blood serum. Interestingly, the osmolality of follicular fluid (275.3 mOsm) was lower than that of serum (282.0 mOsm). There are still no reports about the osmolality of human oviductal fluid, so further research on the osmolality of the in vivo environment of human preimplantation embryos is required. Nevertheless, interestingly, current commercial culture media already have lower osmolality (260-270 mOsm) than the older generation of media (280-295 mOsm) [36].
In the present study, after 3 days of incubation, even under an oil layer, the osmolality of media in the dry culture condition (301.1 mOsm) was higher than that of media in the humid culture condition (285.3–290.4 mOsm) in the dry-type incubator. Gasperin et al. [37] also reported that in the absence of an oil layer, the addition of water in the four-well dish central hole reduced the osmolality of medium (294 mOsm) after 24 hours of incubation compared to the osmolality of control dishes without water (305 mOsm) in a humid-type incubator. These results indicate that osmolality can increase in both dry- and humid-type incubators. Therefore, both an oil layer and extra medium or water supplementation are essential for maintaining the osmolality of the medium by inhibiting evaporation in in vitro culture conditions.
In the present study, in day 5 ET cycles, the rates of clinical and ongoing pregnancy in the humid culture group were higher than the rates in the dry culture group. Similarly, no meaningful difference was found in the clinical and ongoing pregnancy rates of the humid culture groups in day 5 ET cycles (57% and 52%) between the previous [18] and the present study (59.5% and 57.3%), even though there was a large difference in the mean age of female patients between the two studies (28 vs. 36 years, respectively). The first possible reason is medium renewal. We performed medium renewal by exchanging G1 to G2 medium on day 3, but Fawzy et al. [18] did not perform medium renewal for 5 days. Culture for 5 days without medium renewal could degrade the organic osmolytes, and this degradation may augment the negative influence of this osmolality shift on embryo development. In addition, medium renewal may not only reduce the accumulation of metabolites such as ammonium, but could also supply fresh nutrients for embryo development. The second possible reason relates to the humid culture condition. In the present study, for the preparation of the humid culture condition, we added 6 mL of medium into the outer well of GPS culture dishes (LifeGlobal), while Fawzy et al. [18] used petri dishes containing 10 mL of water. In the preliminary experiment, we also used petri dishes containing water, but this was not efficient to inhibit the increase of osmolality in the medium compared to direct medium supplementation in the outer well of the culture dish.
Fawzy et al. [18] reported that the blastulation rate of human embryos was significantly higher in the humid culture group (73%) than in the dry culture group (51%, p<0.05). Gasperin et al. [37] reported a similar trend, according to which the blastocyst rate of bovine embryos was higher in the humid culture group (29.7%, p<0.05) than in the dry culture group (16.2%). Unlike the above results, in the present study, there was no significant difference in the blastulation rate between the dry (56.5%) and humid culture (55.5%) groups. Of particular note, the blastulation rate of the humid culture group (55.5%) was markedly lower than the rate (73%) reported in the other study [18]. This difference may have resulted from the large gap in the mean age of female patients between the other study (28 years) and the present study (36 years). The age of female patients is widely considered as a critical factor for determining the quality of oocytes, the grade of embryos, and the success of pregnancy in IVF-ET cycles. Therefore, the relatively low blastulation rate in the humid culture group in the present study may be explained by the relatively old age of patients compared to the previous study [18].
There was no significant difference in the development of early-stage mouse embryos cultured between the high-osmolality (310–330 mOsm) and low-osmolality (270–290 mOsm) media. However, in the development of late-stage embryos, the embryos cultured in the low-osmolality medium showed a significantly higher blastocyst formation rate than those cultured in the high-osmolality medium [6]. A similar result was observed in porcine embryos cultured in vitro [8]. These results suggest that late-stage embryos are more susceptible to osmotic changes than early-stage embryos. In the present study, a similar pattern in response to osmotic changes in human embryos was observed, although we did not observe a significant difference in the blastocyst formation rate between the high-osmolality (56.5%, dry culture) and low-osmolality (55.5%, humid culture) culture groups. However, in day 3 ET cycles, involving relatively early-stage embryos compared to day 5 ET cycles, the transferred embryos showed a small difference in pregnancy outcomes between the high- and low-osmolality culture groups. The differences in the rates of pregnancy, abortion, and ongoing pregnancy were 4.8%, 6.8%, and 11.7%, respectively. In contrast, in day 5 ET cycles, the differences in these rates in the late-stage embryos were 9.5%, 22.8%, and 32.3%, respectively. This result is consistent with the previous finding that late-stage embryos are more susceptible to osmotic changes [6,8].
When a cell shrinks in hyperosmotic conditions, inorganic ions (Na+, K+) are accumulated via osmotically regulated ion transporters [38]. However, in this case, cells accumulate organic osmolytes intracellularly to replace a portion of the inorganic ions [39-41]. High ionic strength disrupts cell functions, while in contrast, a high concentration of organic osmolytes is not toxic [39,42]. The beneficial action of organic osmolytes was proven by a report that in a hyperosmotic medium (310 mOsm), the blastocyst formation rate of mouse embryos cultured in the presence of 1 mM glycine was significantly more favorable than that of embryos cultured in the absence of glycine [12].
We used G1/G2 medium (Vitrolife), which contains 135–145 µM glycine [43], a concentration that is much lower than the effective concentration of 1 mM in previous studies [12,28]. In the present study, the osmolality of G1 medium was 275 mOsm, which increased to 293.7 and 285.0 mOsm in the dry and humid culture conditions, respectively, after 3 days of incubation. The better clinical outcomes in the humid culture condition than the dry culture condition might have resulted from the low osmolality of the medium in the humid culture condition. Due to the maintenance of low osmolality in the culture medium, glycine probably did not have the chance to act as an organic osmolyte. Instead, the higher osmotic change in the dry culture condition might have increased the concentration of intracellular ionic osmolytes, which are detrimental for embryo development. Moreover, a portion of the ionic osmolytes could not be replaced completely with organic osmolytes because of the low concentration of glycine [43].
In conclusion, the dry culture condition showed a higher osmotic change in the medium than the humid culture condition, even though the media were covered in oil. Hyperosmotic changes showed a detrimental effect on clinical outcomes in human IVF-ET cycles. This hyperosmotic stress could be alleviated by supplementation with outer-well medium to maintain the optimal osmolality of medium, which resulted in the improvement of clinical outcomes in IVF-ET cycles. We are preparing a follow-up study to investigate relationships between the osmolality of culture media and specific patterns of gene expression in porcine embryos.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: **. Data curation: **. Formal analysis: **. Funding acquisition: **. Methodology: **. Project administration: **. Visualization: **. Writing–original draft: **. Writing–review & editing: **.