Management of endometriosis-related infertility: Considerations and treatment options
Article information
Abstract
Endometriosis is a common inflammatory disease in women of reproductive age and is one of the major causes of infertility. Endometriosis causes a sustained reduction of ovarian reserve through both physical mechanisms and inflammatory reactions, which result in the production of reactive oxygen species and tissue fibrosis. The severity of endometriosis is related to ovarian reserve. With regard to infertility treatment, medical therapy as a neoadjuvant or adjuvant to surgical therapy has no definite beneficial effect. Surgical treatment of endometriosis can lead to ovarian injury during the resection of endometriotic tissue, which leads to the deterioration of ovarian reserve. To overcome this disadvantage, a multistep technique has been proposed to minimize the reduction of ovarian reserve. When considering surgical treatment of endometriosis in patients experiencing infertility, it should be kept in mind that ovarian reserve can be reduced both due to endometriosis itself and by the process of removing endometriosis. In cases of mild- to moderate-stage endometriosis, intrauterine insemination with ovarian stimulation after surgical treatment may increase the likelihood of pregnancy. In cases of severe endometriosis, the characteristics of the patient should be considered in a multidisciplinary manner to determine the prioritization of treatment modalities, including surgical treatment and assisted reproduction methods such as in vitro fertilization. The risk of cancer, complications after pregnancy, and infection during oocyte retrieval should also be considered when making treatment decisions.
Introduction
Endometriosis is a chronic inflammatory disease that leads to pain and infertility and occurs primarily in women of reproductive age. For this reason, it is crucial to consider patients’ fertility when treating endometriosis. Previous studies have suggested that roughly 25%– 50% of infertile women have endometriosis, and about 30%–50% of women with endometriosis are infertile [1,2].
Endometriosis is known to contribute to subfertility via pelvic adhesions, distorted pelvic anatomy, and bilateral tubal blockage. In recent studies, immunological, endocrine, biochemical, and genetic derangements, as well as the associated poor quality of the oocyte, embryo, and endometrial environment, have been reported to play a role in the impacts of endometriosis on infertility [3-5].
This review aims to explore the effects of endometriosis on infertility with regard to ovarian reserve, as well as to review in detail the clinical management options for infertile women with different stages of endometriosis.
Effects of endometriosis on ovarian function and its impact on fertility
1. Effects of endometriosis on ovarian function and its impact on fertility
Previous studies have reported that endometriosis damages the ovarian tissue, adversely affecting ovarian function. Endometriosis may disturb physiological reproductive mechanisms, potentially interrupting spontaneous ovulation [6,7]. Endometriosis and associated structural tissue alterations in the ovarian cortex may be a cause of reduced ovarian reserve. Several mechanisms have been suggested to damage ovarian tissue (Figure 1).
The presence of an expanding ovarian cyst may mechanically damage the ovarian tissue, including the follicle; alternatively, it may hamper blood circulation or disturb the vascularization of the tissue through compression of the surrounding ovarian cortex [8]. It has been postulated that the inflammatory reactions typically associated with endometriosis may play a role in damaging ovarian tissue and follicles. Since primordial follicles lack their own vascular network, stromal cells around these follicles are important mediators of nutrient supply and molecular signaling [9-11]. Therefore, the presence of healthy cortex-specific stroma is essential for the development of early follicles. Endometriotic cyst fluid includes high concentrations of toxic substances, such as reactive oxygen species (ROS), inflammatory molecules, proteolytic enzymes, and free iron [12,13]. Previous studies have described the detrimental effects of endometriosis on the adjacent ovarian follicles via altered expression of cytokines, decreased testosterone levels, decreased anti-Müllerian hormone (AMH) levels, and altered expression of lipids and proteins [14-17]. A study by Kim et al. [18] using an animal model reported that endometriotic fluid adversely affected the growth of follicles and the quality of embryos.
Matsuzaki and Schubert [19] reported that the ovarian cortex around endometriomas showed higher levels of oxidative stress than the cortex surrounding other benign ovarian cysts. Endometriomas contain extraordinarily high concentrations of iron compared to serum levels and the follicular fluid of other ovarian cysts. A high concentration of the free or catalytic form of iron in endometriomas mediates the production of ROS via the Fenton reaction [12,20]. Zhang et al. [21] reported that stress from ROS induced oocyte apoptosis and necrosis in early ovarian follicles. Moreover, ROS, along with transforming growth factor beta, is a potent inducer of tissue fibrosis, which also causes follicle loss. Chronic fibrosis causes a progressive decline in ovarian follicle reserve and oocyte quality.
2. Effects of endometriosis on ovarian reserve
As described above, endometriosis damages ovarian tissue through various mechanisms, thereby disrupting the development of ovarian follicles. Consequently, clinical studies have reported the detrimental impact of endometriosis on ovarian reserve. To assess ovarian reserve, the antral follicle count (AFC) has traditionally been used as a clinical indicator. In recent years, assessment of AMH levels has been widely used due to its advantages, including the fact that it can be performed at any time during the menstrual cycle and is unaffected by the use of hormonal medications [22]. To analyze the association between ovarian reserve and the severity of endometriosis, the most commonly used indicator of severity is the American Society for Reproductive Medicine (ASRM) scoring system, which categorizes the disease into four stages; minimal (stage I), mild (II), moderate (III), and severe (IV) [23]. Infertile women are significantly more likely to have moderate to severe endometriosis than previously fertile women [24].
Almog et al. [25] observed a significantly lower AFC in ovaries with unoperated endometriomas than in the contralateral healthy ovaries, and these differences were not observed for other types of benign ovarian cysts. Kim et al. [26] compared an age- and body mass index-matched group of women who underwent surgical resection of ovarian endometriomas with those who had surgically resected mature cystic teratomas of the ovaries. They found that serum AMH levels before surgery were significantly lower in women with severe endometriosis (ASRM IV) than in those with mature cystic teratomas. Shebl et al. [27] adopted the ASRM classification to assess AMH levels according to the severity of endometriosis. The study group was divided into two groups for subgroup analysis: a minimal to mild endometriosis group (ASRM stage I or II) and a moderate to severe endometriosis group (ASRM stage III or IV). In women with minimal to mild endometriosis, serum AMH levels were not significantly different from those in the healthy control group, whereas women with moderate to severe endometriosis showed statistically significantly lower AMH levels. Taken together, the results of previous studies indicate that ovarian endometriosis reduces ovarian reserve, especially in severe cases.
Kasapoglu et al. [28] evaluated whether the endometriosis-associated decline in ovarian reserve is progressive in the absence of treatment and greater in magnitude than the natural decrease over time. They found that endometriosis was associated with a faster decline in serum AMH levels, which are a marker of ovarian reserve, than the decline observed in healthy controls. Previous studies have suggested that women with ovarian endometriomas not only have reduced ovarian reserve, but also have a steeper of ovarian reserve decline over time. Reflecting these results, women with a history of endometriosis or endometriosis-related infertility are likely to experience menopause earlier than those without such a history [29,30].
3. Effects of endometriosis on fertility
In addition to the reduction of ovarian reserve, distortion or obliteration of the functional adnexal structure may act as a physical barrier that contributes to the induction of subfertility. Moreover, it is thought that endometrial progesterone resistance, increased cell proliferation, and decreased cell apoptosis, which are caused by dysregulation of the biomarkers involved in endometrial receptivity, ultimately contribute to subfertility [31,32].
A causal relationship between endometriosis and infertility has been established through the application of evidence-based principles [33]. A large cohort study of women under 35 years of age showed that women with endometriosis were twice as likely to experience infertility than women without endometriosis [34]. The monthly fecundity rate of women with endometriosis has been reported to be 2%–10%, which is lower than the rate of 15%–20% reported for healthy women, and the greater the severity of the disease, the lower the fecundity [24,35]. With no intervention, about 50% of women with mild endometriosis can conceive; of those with moderate endometriosis, only 25% can conceive, and only a few with severe disease can conceive [36]. As such, the severity of endometriosis is related to the probability of pregnancy.
Medical treatment options in infertile patients with endometriosis
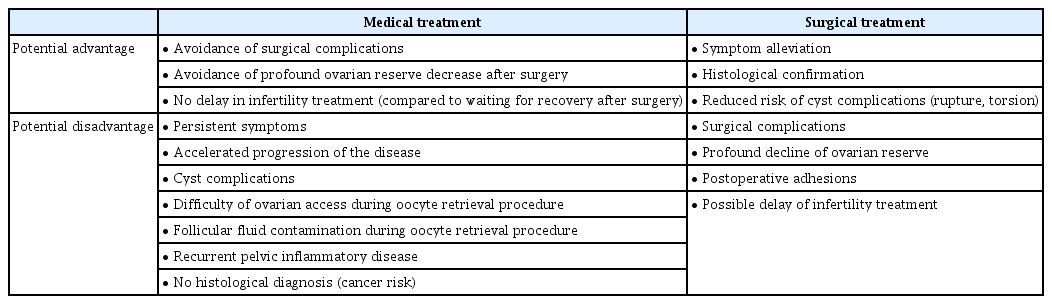
Both medical and surgical treatment options for endometriosis exist. The potential benefits and disadvantages of medical and surgical treatment are summarized in Table 1. Medical treatment options include oral contraceptives, progestins, aromatase inhibitors, and gonadotropin- releasing hormone (GnRH) agonists. These medical treatments are based primarily on methods that inhibit ovarian function. As a result, medications administered for this purpose usually provoke contraception or subfertility, and these medical treatments therefore are not helpful for treating endometriosis-associated infertility with the goal of improving pregnancy outcomes, including live births [37]. Medical therapy as a neoadjuvant or adjuvant to surgical therapy has not been reported to be effective for infertility treatment [38,39].
Surgical treatment options in infertile patients with endometriosis
Surgical interventions can be performed through laparoscopy, laparotomy, or robotic surgery. Due to its associated advantages, including a shorter recovery time and lower costs, the laparoscopic approach is currently the most widely accepted method for the diagnosis and resection of endometriotic lesions [40]. To alleviate the deleterious environment caused by endometriosis, some believe that it may be beneficial to perform surgical treatment even at an early stage of cyst development [41]. However, routine surgical treatment is generally not recommended.
1. Effects of ovarian surgery on ovarian reserve
Surgical treatment of endometriosis is associated with the possibility of ovarian injury. This has been a major concern regarding surgery as a treatment modality, and various attempts have been made to minimize the associated damage to ovarian tissue [42,43]. The stripping technique for endometrioma excision may cause surgical injury to normal ovarian tissue [44,45]. Removal of excessive ovarian tissue along with the walls of ovarian cysts may cause follicle loss [46]. Muzii et al. [47] compared surgically resected endometriotic cysts with other types of ovarian cysts (dermoids and serous or mucinous cysts) to evaluate this phenomenon. In 54% of cases of endometrioma resection, the ovarian tissue was removed—a higher frequency than observed for other types of ovarian cysts (6%). In terms of pathogenesis, the endometrioma cyst wall is a pseudo-capsule; therefore, healthy ovarian tissues are also inadvertently removed during the stripping process of the endometrioma in laparoscopic surgery.
Electrical coagulation of the remaining parenchyma of the ovary after excision of the cyst wall may cause thermal damage, resulting in a decrease in AMH levels immediately after surgery [48]. Further damage to the ovarian tissue can affect vascular structures, compromising circulation [49]. The factors that affect the decrease of ovarian function by 1 week postoperatively are inflammation, edema, vascular injury, and ischemia [50,51]. The long-term progressive decline observed thereafter can be attributed to impaired vascularization [52-54].
In a prospective study of patients undergoing laparoscopic unilateral cystectomy, Lee et al. [55] found that serum AMH levels significantly decreased immediately after surgery and remained low for up to 3 months. Two systematic reviews published in 2012 reported the impact of surgical excision of endometrioma on ovarian reserve. Raffi et al. [56] analyzed eight prospective studies and documented that postoperative serum AMH levels significantly decreased (38%) following surgery. In patients undergoing unilateral endometrioma cystectomy, serum AMH levels decreased by 30%, while a 44% reduction was observed after bilateral endometrioma surgery. Uncu et al. [57] prospectively compared AMH levels after bilateral cystectomy and unilateral cystectomy and found that AMH levels were significantly lower in the former group at 6 months postoperatively. Eleven studies were reviewed by Somigliana et al. [58], of which nine reported a significant decline in serum AMH levels following surgery. In that study, the presence of bilateral endometriomas, preoperative serum AMH levels, and the presence of healthy ovarian tissue adjacent to the wall were determined to be significant factors related to decreased AMH levels.
2. Effects of ovarian surgery on fertility
When evaluating the effect of fulguration of endometriotic implants in patients with mild endometriosis, operative laparoscopy was found to be superior to simple diagnostic laparoscopy with regard to the spontaneous pregnancy rate [59]. In the treatment of minimal and mild endometriosis (ASRM I and II), the use of laparoscopic surgery (excision or ablation of the endometriotic lesions) may improve the spontaneous pregnancy rate and live birth rate compared to expectant management [60,61].
In comparisons of methods of laparoscopic management of ovarian endometriomas larger than 3–4 cm, excisional surgery was shown to yield more favorable outcomes than drainage and ablation with regard to subsequent spontaneous pregnancy in subfertile women [62,63]. In cases of moderate to severe (ASRM III or IV) endometriosis, surgery can be useful to treat pelvic adhesions that may hamper reproductive mechanisms. However, there is a lack of randomized controlled trials on postoperative pregnancy outcomes in these patients, so a robust consensus has not yet been established. In infertile women with endometriosis, the cumulative probability of pregnancy at 3 years after surgery was 47% (women who underwent in vitro fertilization [IVF] cycles were excluded from this analysis), and there was no significant difference in the pregnancy rate according to ASRM stage [64]. This pregnancy rate was much higher than the crude pregnancy rates of 33% (moderate endometriosis) and 0% (severe endometriosis) after expectant management [36,65]. Considering these results, in infertile women with moderate to severe endometriosis, clinicians can consider surgery as an alternative to expectant management. In such cases, clinicians should consider the possibility of reduced ovarian function after surgical treatment, and patients should be made aware of this possibility.
3. Conservative surgical management to minimize ovarian damage
Thermal insult caused by electrical cauterization is the main mechanism by which cauterization damages the ovarian tissue and vascular structure, inducing long-term ischemic impairment. The use of bipolar cauterization for hemostasis after endometrioma resection from the ovarian bed appears to damage ovarian reserve more than the use of sutures or hemostatic sealants [66,67]. Therefore, surgeons should strive to minimize thermal damage caused by bipolar cauterization. When performing surgery, the concave area should be clearly exposed, and blind coagulation should be avoided. When performing coagulation near the area of the hilus, where there are many blood vessels, caution is necessary.
As shown in previous studies, ovarian tissue is unintentionally removed in many cases of endometriotic cyst resection [47]. For this reason, when performing a laparoscopic surgical intervention, tissuesparing procedures should be implemented to protect the follicular reserve of the ovarian cortex. In line with this objective, Donnez et al. [44] proposed a method to remove the part of the cyst farther from the hilus using the conventional cystectomy technique and to remove the remaining 10%–20% of the endometrioma wall from the hilus using a CO2 laser. However, this combined technique did not have a beneficial effect on ovarian reserve as evaluated by ovarian volume and AFC.
Beretta et al. [68] compared cystectomy with concurrent drainage and coagulation of endometriomas, and their study revealed that cystectomy resulted in longer-lasting pain relief and a lower recurrence rate of dysmenorrhea, deep dyspareunia, and non-menstrual pelvic pain. Var et al. [69] conducted a prospective randomized trial of women with bilateral endometriomas to compare excision and coagulation. Six months after surgery, decreases in AFC and ovarian volume were found for both coagulation and cystectomy; however, the decrease was statistically significantly greater for cystectomy than for coagulation. As such, these results are contradictory regarding which method is superior.
Two-step or three-step surgical techniques have been suggested to minimize the harmful effects of the conventional stripping technique. In such techniques, drainage of the cystic fluid, irrigation, and biopsy are performed as part of the first procedure. For the next 3 months, a GnRH agonist is provided to suppress the endometriotic lesion. This procedure generally decreases the cyst diameter to about 50% of its previous size. Three months after first-look surgery, the second-look laparoscopic procedure was performed, and the inner wall of the cyst was vaporized with a CO2 laser [70]. This two-step (or three-step if we consider GnRH-agonist treatment to be an individual step) procedure has been performed by several surgeons, and Tsolakidis et al. [71] compared the impact of cystectomy versus the three-step procedure on ovarian reserve in endometrioma patients via a prospective randomized trial. After 6 months, the serum AMH levels were significantly reduced in the cystectomy group, but not in the three-step procedure group. The AFC was significantly higher following the three-step procedure, but did not significantly change after cystectomy .
When considering minimally invasive surgery for endometrioma, the recurrence rate after surgical treatment should be kept in mind. Abbott et al. [72] reported that the probability of second-line surgery was 36% at about 2 to 5 years post-laparoscopic excisional surgery. In a retrospective analysis of recurrent endometriomas after unilateral ovarian surgery, it has been reported that a higher recurrence rate was associated with higher ovarian reserve [73]. This multi-step procedure seems to avoid removal of normal ovarian tissue and reduce thermal damage by the use of laser vaporization, resulting in better preservation of the ovarian follicle pool. However, it is unclear whether these factors increase the recurrence rate of endometriosis. Considering the heterogeneity and the limitations of the few available existing studies, additional well-designed trials are needed to address this complicated issue.
Outcomes of assisted reproductive techniques in infertile patients with endometriosis
1. Intrauterine insemination
Intrauterine insemination (IUI) is a relatively simple procedure compared to IVF, and a number of studies have been reported of couples with minimal to mild endometriosis and normal semen quality. In patients with surgically diagnosed and treated ASRM I or II endometriosis, controlled ovarian stimulation (with clomiphene citrate or gonadotropins) and IUI may be a reasonable treatment alternative to IUI alone, IVF, or further surgical therapy [2,74,75]. Infertile patients with minimal to mild endometriosis displayed a lower pregnancy rate after controlled ovarian stimulation and IUI than women diagnosed with unexplained infertility [76]. After surgical treatment, the clinical pregnancy rate per cycle and the cumulative live birth rate were similar between the endometriosis and unexplained infertility groups [77].
In treating infertile women with endometrioma, compared to ablative surgery, excisional surgery resulted in increased ovarian follicular response to gonadotropin stimulation. However, there is not enough evidence to support excisional surgery over ablative surgery with respect to the possibility of pregnancy after controlled ovarian stimulation and IUI [62]. IUI is not typically attempted in women with moderate to severe endometriosis due to concerns for pelvic adhesion and decreased tubal function. In these cases, IVF should be considered.
2. In vitro fertilization
As noted above, ovarian endometriosis appears to reduce ovarian reserve, and thus, the ovarian response to gonadotropin stimulation may be decreased in cases of endometriosis. Somigliana et al. [78] reported that women with unilateral ovarian endometriomas showed reduced ovarian responsiveness when controlled ovarian hyperstimulation was performed as part of the IVF cycle. Ovarian responsiveness was significantly associated with the size and number of endometriomas. However, other findings contradict this result. A retrospective analysis of IVF treatment cycles showed that the number of antral follicles and total number of oocytes retrieved in women with unilateral endometriomas were not significantly different from those without endometriomas or endometriosis. There was also no association between the size or number of endometriomas and the number of oocytes retrieved [25,79].
A recent meta-analysis conducted by Hamdan et al. [80] reported that women with endometriomas undergoing IVF without surgical management had similar clinical pregnancy rates and live birth rates to those without the disease, although the mean number of oocytes retrieved was lower and the cycle cancellation rate was significantly higher in women with endometriomas.
Moderate to severe endometriosis appears to have adverse effects on the outcomes of IVF; however, there is no clear conclusion as to whether to perform surgery on these patients prior to IVF due to the lack of randomized controlled trials, the inconsistent results of previous studies, and the large variability in methodology between studies [80]. Some authors have demonstrated that surgical excision of endometrioma had no detrimental impact on ovarian response in IVF cycles [81,82]. However, Benaglia et al. [83] reported contradictory results regarding controlled ovarian stimulation for IVF in 93 women who underwent unilateral endometrioma resection. Among them, 12 had no follicular growth in the ovary of the operated side, whereas follicular growth was observed in the contralateral ovary .
According to Hong et al. [84], there was no difference in the IVF outcomes between women with diminished ovarian reserve after endometrioma surgery and those with diminished ovarian reserve without previous ovarian surgery. A systematic review comparing artificial reproductive technology (ART) outcomes between women who had endometriomas with no surgical treatment and those who underwent endometrioma excision prior to IVF revealed similar clinical pregnancy rates, live birth rates, and mean numbers of retrieved oocytes [80].
When treating infertile women with endometriosis, there is no evidence that cystectomy of cysts larger than 3 cm prior to treatment with ART improves pregnancy rates [38,62,85,86]. The effectiveness of surgical excision of deep-infiltrating endometriosis before undergoing ART is not conclusive [87,88].
Vercellini et al. [89] performed a systematic review of IVF results following second-line conservative surgery for recurrent ovarian endometriomas after primary conservative surgery. The probability of pregnancy after repeat surgery for recurrent endometriosis seems to be lower than that after primary surgery. In a recent study by Park et al. [90], the numbers of oocytes retrieved after IVF, mature oocytes, and high-grade embryos were significantly lower in the second-line surgery group. These results suggest that second-line conservative surgery appears to significantly reduce ovarian reserve. Accordingly, even if endometriosis recurs, second-line conservative surgery should be carefully considered when treating women with the desire to conceive.
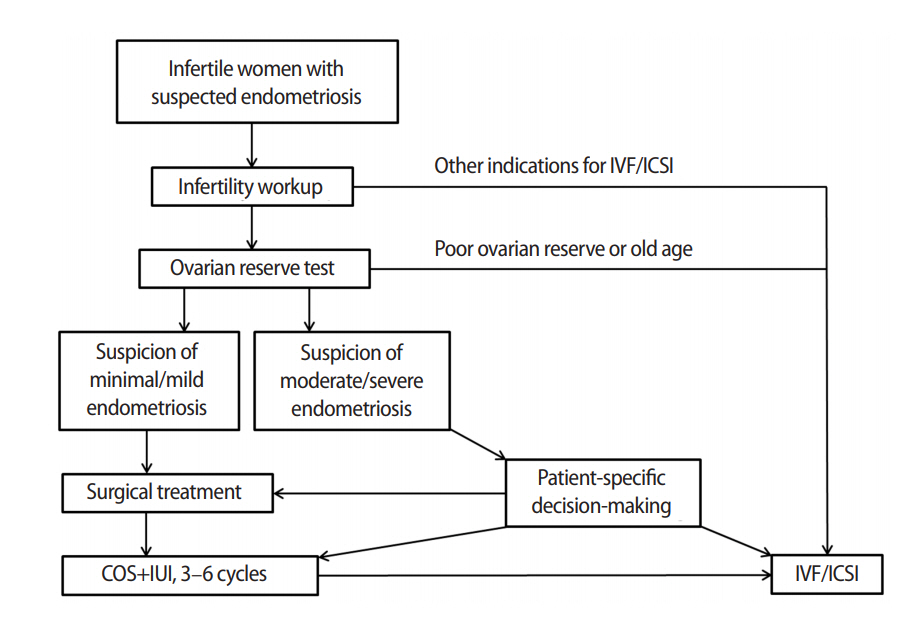
In conclusion, surgical treatment and subsequent IUI with ovarian stimulation should be considered in women with minimal to mild endometriosis with infertility. However, in women with moderate to severe endometriosis, surgical treatment can reduce ovarian reserve and adversely affect subsequent IVF outcomes. According to the recent European Society of Human Reproduction and Embryology guidelines, surgical excision is not routinely recommended before considering ART [38]. Clinicians are advised to assess ovarian reserve prior to deciding whether to surgically remove endometriotic lesions. It is recommended to measure the AMH level at least 3 months postoperatively to decide on an optimal treatment plan. Individualized care provided with this in mind to women with endometriomas prior to IVF may help optimize their pregnancy outcomes. Based on the findings so far, the algorithm for the treatment of infertile women with endometriosis is summarized in Figure 2. The criteria for specific values of ovarian reserve markers used in decision-making regarding surgical treatment have not yet been established, and further research should be conducted.
Other issues to consider when treating infertile women with endometriosis
1. Risk of ovarian cancer
According to a large population-based contemporary study, the 5-year risk of cancer was not increased among women of reproductive age who previously underwent ART treatment. However, many retrospective and prospective studies have documented an association between ovarian endometrioma and ovarian cancer of clear cell and endometrioid histology [91]. Therefore, even if the probability is low, the clinician should treat patients with due consideration of the possibility that an endometrioma may be cancer or may progress to cancer.
2. Risk of complications during pregnancy
Previous studies have suggested that endometriosis can negatively affect the physiological development of pregnancy and may cause pregnancy-related complications. Delayed implantation can result in placenta previa or placental insufficiency, which can lead to intrauterine growth restriction, pre-eclampsia, and obstetric hemorrhages. Endometriosis-associated implantation beyond the normal implantation window can also cause spontaneous abortion and recurrent pregnancy loss. Premature decidual senescence can lead to preterm birth [92]. Although the results of epidemiological studies are contradictory, some evidence is suggestive of an association between endometriosis and miscarriage, placenta previa, preterm delivery, and small-for-gestational-age babies. There is currently no evidence that prophylactic surgery prevents the negative effects of endometriosis on pregnancy outcomes.
3. Risk of disease progression during IVF cycles
Endometriosis is an estrogen-dependent disease, and there may be concerns about whether controlled ovarian stimulation for ART affects the recurrence or disease progression of endometriosis. Patients treated with ART displayed a cumulative recurrence rate similar to that of the control group, and gonadotropin treatment did not seem to affect the natural history of endometriotic lesions [93]. Likewise, it has been found that temporary exposure to very high estrogen levels during controlled ovarian stimulation for IVF is not a risk factor for endometriosis recurrence [94].
As a consequence of the successes of ART, pregnancy in mothers with existing endometriosis has emerged as a subject of interest. Ueda et al. [95] reported that in such cases, the size of the cyst decreased in 52% of patients, was unchanged in 28%, and increased in 20%. The endometriotic lesions demonstrated decidualization, abscess, and rupture. In other studies, most lesions did not diminish or change in size; thus, endometriosis can be conservatively observed during pregnancy [96].
4. Risk of contamination or infection during IVF cycles
Endometriomas contain several factors thought to be toxic for oocytes, including metalloproteinases, cytokines, free iron, and ROS [12]. However, direct evidence regarding the detrimental effects of incidental follicular fluid contamination is scant and contradictory. Small-scale studies suggest that such contamination may reduce the fertilization rate or the pregnancy rate [97,98], thus rendering it necessary to avoid inadvertent puncture of the endometrioma and to wash the oocyte immediately if contamination is confirmed.
An iatrogenic injury after oocyte retrieval or inadvertent puncture during oocyte retrieval may cause infection in cases of ovarian endometrioma. Until recently, only 14 cases of infection after oocyte retrieval have been reported in nine studies, although it is possible that this complication is underdiagnosed [99]. However, it is of clinical importance that the endometriotic fluid itself can act as a culture medium for pathogens, and because antibiotics do not spread effectively into endometriomas, they may progress to become severely infected. Prophylactic antibiotics do not completely prevent such infections, but if puncture of the endometrioma is suspected, it is necessary to reduce the probability of infection by using of antibiotics.
Conclusion
The management of endometriosis-related infertility remains challenging and is a subject of debate. Endometriosis has been shown to induce infertility through a variety of mechanisms, and the reduction of ovarian reserve is one of the factors most directly involved in decreased fertility. Endometriosis itself contributes to the reduction of ovarian reserve, and surgical treatment of endometriosis can also damage ovarian reserve. Therefore, when considering surgical treatment, it is important to make decisions with due consideration of the severity of each case of endometriosis and the unique characteristics of each patient, and it is also important to share opinions with patients in this process. Medical treatment alone for endometriosis has little effect on infertility treatment, and the effects of therapy adjuvant or neoadjuvant to surgical treatment are unclear.
Insufficient evidence is available to confirm that surgery is a reasonable option for the treatment of infertility associated with endometriosis. Medical or hormonal therapy should be used as an adjunct to ART because such therapies themselves have little effect on infertility. IUI is the first-line method of choice after surgical treatment of minimal to mild endometriosis because of its ease of administration. In patients with severe endometriosis, IVF can be considered as a treatment option. Physicians should accurately diagnose patients’ conditions and provide personalized treatment plans, while taking into account the advantages and disadvantages of each treatment option.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: SKK. Writing - original draft: DL. Writing - review & editing: SKK, JRL, BCJ.