Delayed recovery of a patient with obstructive azoospermia and a history of acute epididymitis
Article information
Abstract
Obstructive azoospermia caused by acute epididymitis is usually permanent, and microsurgical vasoepididymostomy is the only reconstructive treatment option. There have been no reports of delayed recovery of sperm count after over 1 year in a patient with obstructive azoospermia related to history of acute epididymitis. We present a young male patient who had azoospermia and a history of acute epididymitis who experienced delayed recovery, with complete restoration of sperm production and the ability to conceive naturally.
Introduction
Obstructive azoospermia is a urologic condition that occurs in fewer than 1% of all men, but in approximately 10%–15% of infertile men [1]. Obstructive azoospermia is caused by an anatomical block in the epididymis, vas deferens, or ejaculatory duct in the prostate gland. The underlying etiology may be infection, iatrogenic injury, or a genetic or congenital condition. Acute epididymitis is a common genitourinary condition that may lead to a transient deterioration of sperm quality or permanent epididymal and vasal obstruction [2]. Pathogens can induce intense inflammatory reactions, resulting in secondary scarring and obstruction of the epididymis and vas deferens [3]. Obstructive azoospermia from acute epididymitis usually does not resolve without surgical treatment. Microsurgical vasoepididymostomy is the only available reconstructive surgical treatment for obstructive azoospermia secondary to acute epididymitis. No previous study has reported the delayed recovery of sperm count more than 1 year after treatment in a patient with obstructive azoospermia secondary to acute epididymitis. Here, we present a case of a young man with obstructive azoospermia associated with a history of acute epididymitis who experienced delayed recovery, and then produced sufficient sperm for natural conception.
Case
A 20-year-old male patient visited our andrology clinic for a fertility evaluation. He had engaged in regular sexual intercourse with his 20-year-old female partner without contraception for 6 months, but she did not get pregnant. Twelve months previously, he had received 3 weeks of treatment with oral antibiotics for right acute epididymitis. He had no chronic medical diseases, took no daily medications, had no history of trauma to the scrotum or genitalia, and had no symptoms related to testosterone deficiency. His libido, erectile function, and ejaculation volume were normal. The physical examination indicated he had a healthy and normal male appearance, a body height of 174 cm, and a body mass index of 23.1 kg/m2. He had a normal male pattern of hair on the face, body, axillary regions, and pubic regions, and did not have gynecomastia. Examination of the external genitalia indicated they were normal, and the stretched penis length was 13 cm. Both testes were normally positioned in the scrotum, had normal consistency on palpation, and were normal in size (20 mL each).
No abnormalities of the epididymis and vas deferens were noted. Semen analysis showed azoospermia, with a volume of 1.4 mL and small amounts of inflammatory cells (0.7×106/mL; normal range, <1×106/mL). There were normal levels of serum follicle-stimulating hormone (4.48 mIU/mL; normal range, 1.5–12.4 mIU/mL), luteinizing hormone (4.29 mIU/mL; normal range, 1.24–8.62 mIU/mL), prolactin (14.3 ng/mL; normal range, 4.04–15.2 ng/mL), and testosterone (3.40 ng/mL; normal range, 2.49–8.36 ng/mL). A second semen analysis 2 weeks later also showed azoospermia with volume of 1.7 mL. There was no sperm in the post-ejaculatory urine.
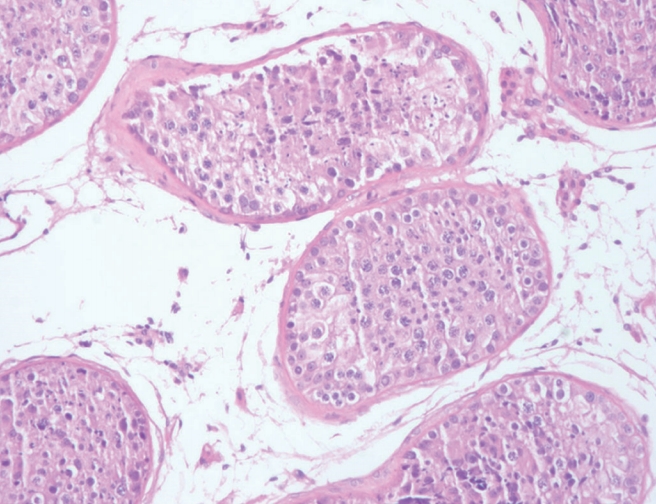
We performed ultrasonography of the scrotum and prostate. Doppler ultrasound of the scrotum indicated normal size, echogenicity, and blood flow of the testis and epididymis, and no definite tubular ectasia of the rete testis or the epididymis (Figure 1). Transrectal prostate ultrasonography showed no obstructive lesion in the prostate and no seminal vesicle dilatation or ejaculatory obstruction, but a slightly enlarged prostate volume of 24 mL (Figure 2). To confirm testicular spermatogenesis, we performed a biopsy of the left testis under local anesthesia, and the result indicated normal spermatogenesis in the seminiferous tubules (Figure 3). Our final diagnosis was obstructive azoospermia related to a history of acute epididymitis.
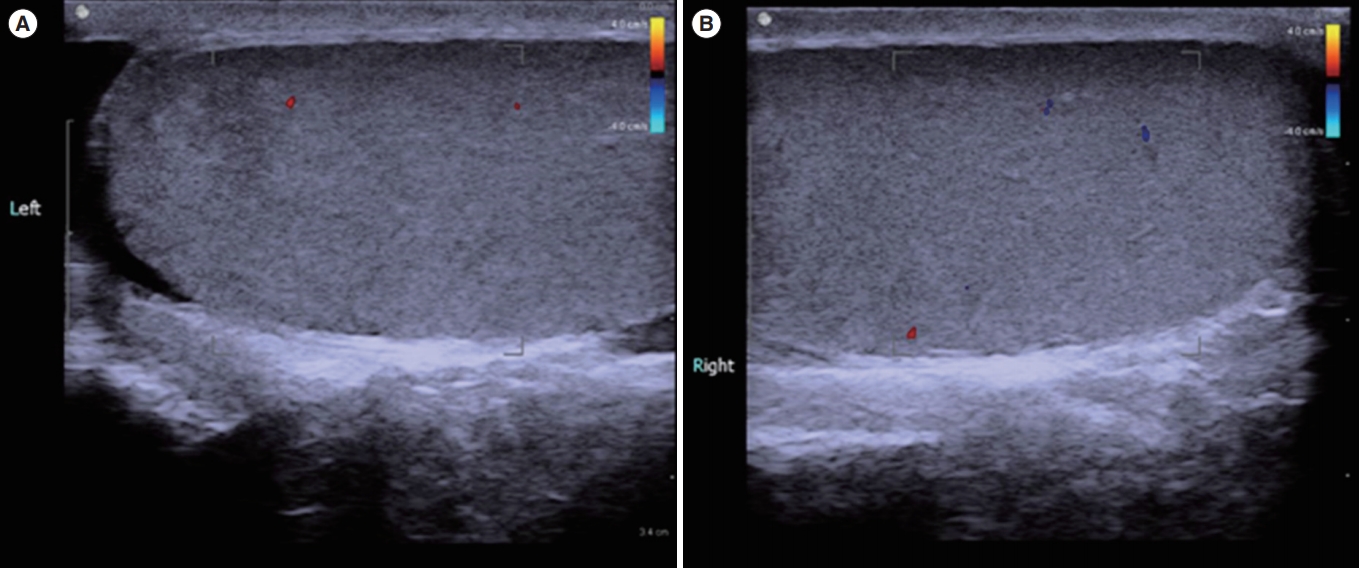
Doppler scrotal ultrasonography showed normal size, echogenicity and blood flow of both testis (A, left testis; B, right testis).

Transrectal prostate ultrasonography showed normal echogenicity and blood flow of prostate without any obstructive lesion.
The patient was lost to follow-up until he visited again 18 months later because his female partner had become pregnant. A follow-up semen analysis indicated normal semen volume (3.8 mL; normal range, ≥1.5 mL), sperm concentration (35×106/mL; normal range, ≥15×106/mL), motility (47%; normal range, ≥40%), and strict normal morphology (4%; normal range, ≥4%). We recommended an additional semen analysis 1 month later, but the patient was lost to further follow-up.
Discussion
We describe a young male patient with azoospermia and a history of acute epididymitis who experienced delayed recovery, as indicated by adequate sperm production and the ability to conceive naturally. Acute epididymitis can have several etiologies, including bacterial ascent, viral and fungal infection, drug-induced epididymitis, rheumatic disease, trauma, and sterile urine reflux [2]. Bacterial ascent through the urogenital tract plays a important role in the etiology of acute epididymitis, with common microorganisms including typical uropathogens such as Escherichia coli and sexually transmitted pathogens [4]. Nicholson et al. [5] reported that the annual incidence of acute epididymitis was approximately 400 per 100,000 men. The symptoms of acute epididymitis are typically unilateral scrotal pain and epididymal swelling. Epididymitis can also be asymptomatic [6]. Bilateral involvement is reported to occur in 4% of patients. Because 60% of patients with acute epididymitis show testicular involvement, many researchers use the term epididymo-orchitis rather than epididymitis [7]. Urogenital infections, including acute epididymitis, can have various effects on male fertility. These include direct or indirect damage to sperm quality and function by the pathogens themselves or reactive oxygen species, inflammation-related obstruction of the reproductive tract, inhibition of spermatogenesis through direct effects of the pathogens or their components, and induction of a humoral immune response to sperm [8]. The deterioration of sperm concentration in the ejaculate during acute epididymitis is usually reversible within 3 to 6 months [8]. However, acute epididymitis can cause an intense inflammatory reaction, leading to secondary scarring and obstruction of the epididymis; therefore, reduced sperm quality or azoospermia can remain permanent even after treatment. Rusz et al. [9] reported in a systematic review that after treatment of urogenital infections, persistent azoospermia occurred in approximately 10% of patients and oligozoospermia in another 30% of patients.
The mechanism of obstructive azoospermia in unilateral epididymitis is not clear. In acute epididymitis, complete obstruction of the epididymis as well as of the vas deferens or the ejaculatory duct may develop as a result of bacterial ascent through the urogenital tract. However, acute epididymitis can be asymptomatic [6], and subclinical contralateral epididymitis might be present in patients diagnosed with unilateral epididymitis. In a small study of biopsy findings, Osegbe reported that patients presenting with unilateral acute epididymitis who underwent contralateral testicular biopsy showed evidence of gonadal damage and azoospermia in the contralateral testis, suggesting the presence of subclinical bilateral involvement [10].
A transient state of azoospermia may be caused not only by urogenital infection, but also by toxic, environmental, or iatrogenic factors such as testosterone replacement therapy [11]. Nutritional deficiencies, heat exposure, and various endocrinopathies can lead to transient azoospermia [12]. Temporary azoospermia can also occur following systemic infections, and can last for 2 to 3 months [11]. Odom and Kutteh [13] reported a case of azoospermia secondary to an acute febrile episode from meningitis, in which the sperm count recovered 5 months later with a successful natural pregnancy.
If azoospermia continues for more than 6 months after treatment for acute epididymitis, then obstructive azoospermia due to secondary scarring and obstruction of the epididymis is likely, and microsurgical vasoepididymostomy could be considered as a treatment option. There have been no previous reports of delayed recovery from obstructive azoospermia secondary to acute epididymitis more than 12 months after medical treatment. The mechanism of late recovery of azoospermia in our patient is uncertain. Because pathogenic bacterial ascent through the urogenital tract is the most important cause of acute epididymitis, it is possible that acute epididymitis occurred together with acute prostatitis, potentially resulting in swelling of the epididymal tubules and ejaculatory duct. The transient obstruction that may have resulted from edema of the small tubules of the epididymis and the ejaculatory duct following an epididymal and/or prostatic infection could take a relatively long time to resolve, even over 1 year. If this hypothesis is correct, it could explain this patient’s very delayed recovery from azoospermia more than 1 year after acute epididymitis. The limitations of this case report include the lack of semen analysis at the time of acute epididymitis treatment and the absence of medical information regarding the patient’s contralateral epididymal status or the possibility of a concomitant prostate infection in the acute phase of epididymitis. This is a rare case report of delayed recovery from obstructive azoospermia that occurred more than 1 year after acute epididymitis treatment.
Notes
Funding
*This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1G1A1002237).
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: SHS, SS, DSK. Data curation: JYS, YSH, MO, DHS, WSL, JB. Formal analysis: SHS, DSK. Funding acquisition: DSK. Methodology: SHS, JL. Project administration: SHS. Visualization: DSK. Writing - original draft: SHS, DSK. Writing - review & editing: DSK.
Acknowledgements
We would like to thank Duygu Unuvar Purcu (Biologist) for technical help in some experiments.