The effectiveness of earlier oocyte retrieval in the case of a premature luteinizing hormone surge on hCG day in in vitro fertilization-embryo transfer cycles
Article information
Abstract
Objective
To evaluate the efficacy of earlier oocyte retrieval in IVF patients with a premature LH surge on hCG day.
Methods
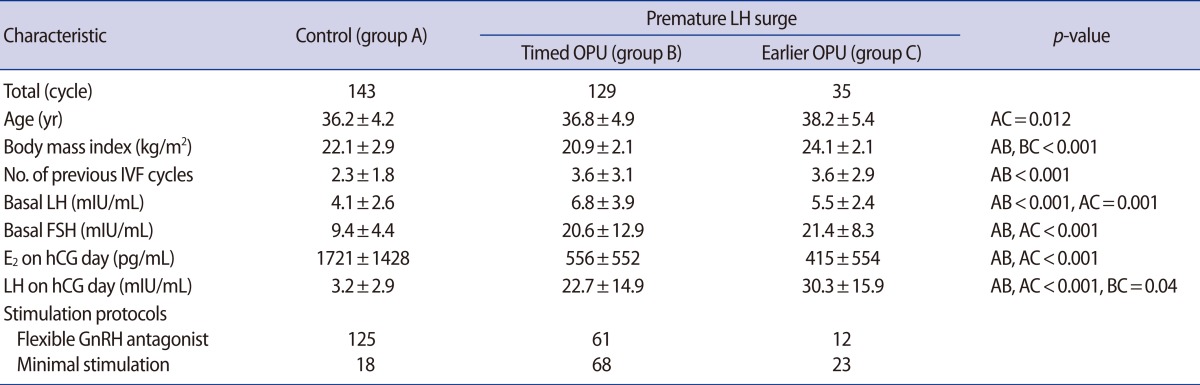
One hundred forty IVF patients (164 cycles) with premature LH surge on hCG day were included, retrospectively. We divided them into 2 study groups: LH surge with timed ovum pick-up (OPU) 36 hours after hCG injection (group B, 129 premature cycles), and LH surge with earlier OPU within 36 hours after hCG injection (group C, 35 cycles). Control groups were tubal factor infertility without premature LH surge (group A, 143 cycles).
Results
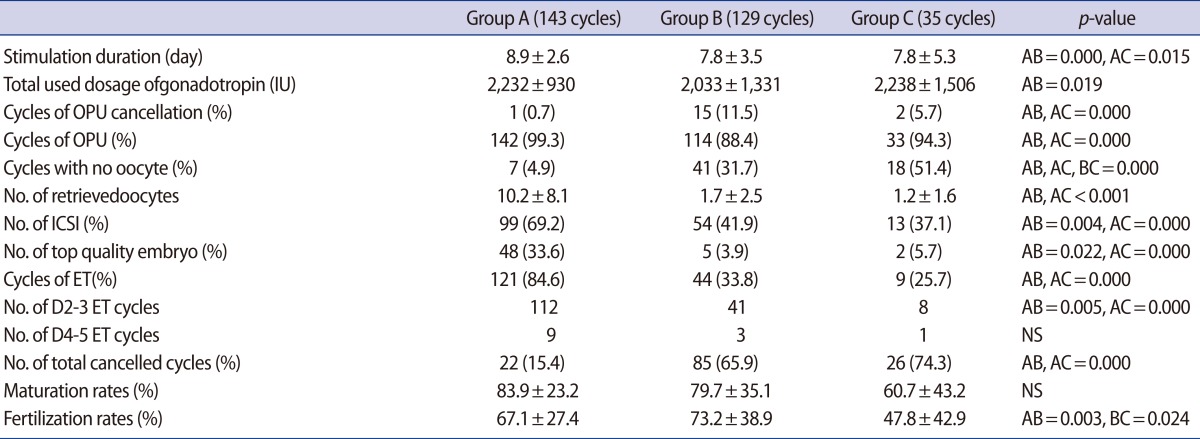
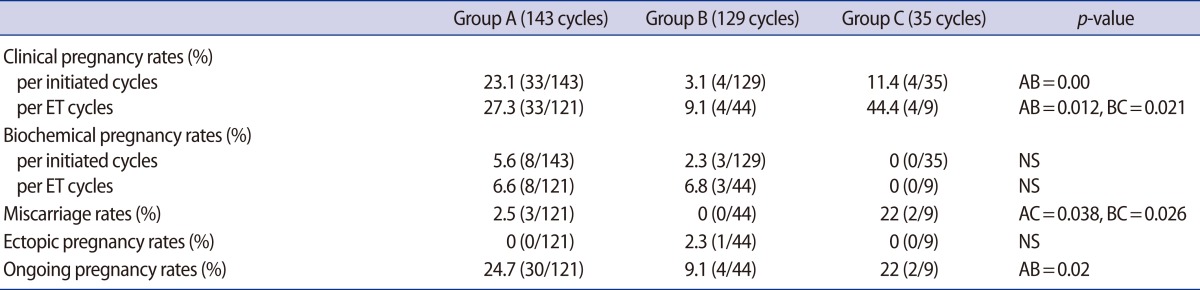
The mean age (year) was statistically higher in group C than in groups A or B (38.2±5.4 vs. 36.2±4.2 vs. 36.8±4.9, respectively; p=0.012). The serum LH levels (mIU/mL) on hCG day were significantly higher in group B and C than in group A (22.7±14.9 vs. 30.3±15.9 vs. 3.2±2.9, respectively; p>0.001). Among groups A, B, and C, 4.9%, 31.7%, and 51.4% of the cycles, respectively, had no oocytes, and the overall rates of cycle cancellation (OPU cancellation, no oocyte, or no embryos transferrable) were 15.4%, 65.9%, and 74.3%, respectively. The fertilization rate (%) was significantly higher in group B than in group C (73.2±38.9 vs. 47.8±42.9, p=0.024). The clinical pregnancy rate was significantly higher in group C than in groups A and B (44.4% vs. 27.3% vs. 9.1%, respectively, p=0.021). However, the miscarriage rate was also higher in group C than in group B (22% vs. 0%, respectively, p=0.026).
Conclusion
Earlier OPU may not be effective in reducing the risk of cycle cancellation in patients with premature LH surge on hCG day. A larger scale study will be required to reveal the effectiveness of earlier ovum retrieval with premature LH surge.
Introduction
LH is produced by gonadotropic cells in the anterior pituitary gland. In females, a sharp rise in LH triggers ovulation and development of the corpus luteum. LH is similar molecularly to hCG, in that the first 114 amino acids of each compound share 80% homology. They also bind to the same hormone receptor [1].
Exogenous gonadotropin is needed to replace the endogenous LH surge in controlled ovary hyperstimulation (COH). The most commonly used exogenous LH is hCG, which simulates the physiologic effects of LH, and is used to trigger the final follicular maturation before oocyte retrieval in assisted reproductive technology assisted reproductive technology (ART) programs [2]. In many studies, the 36-hour interval to hCG administration and oocyte retrieval has been found to have the greatest effectiveness with regard to the fertilization rate and pregnancy rate [3-6].
In some studies, the IVF cycle cancellation was 1.2% because of premature luteinization despite the use of GnRH antagonist to prevent an endogenous LH surge [7,8]. Generally, a premature LH surge is defined as a serum LH level of ≥10 mIU/mL or an increase of more than 2.5 times above the basal level [9-11]. The occurrence of a premature LH surge during controlled ovarian stimulation (COH) is associated with reduced pregnancy rates [10,12,13]. However, there is no clear-cut threshold to use as an indication to cancel an IVF cycle when a premature LH surge has occurred [14]. Moreover, it is unclear whether the significant factor in these LH surges is the ascent of LH or the subsequent descent. If the descent following an LH surge were the relevant factor, we could not retrieve oocytes through timed ovum pick-up (OPU). An earlier OPU would thus be more effective for retrieving oocytes in order to avoid cancelling the cycle.
The purpose of this study was to compare the outcomes of ART between normal LH status and premature LH surge cycles on hCG day and to evaluate the benefit of earlier ovum retrieval in the case of a premature LH surge on hCG day.
Methods
1. Study population
This retrospective study included a total of 307 cycles (266 IVF patients). Among them, the 140 IVF patients (164 cycles) with a premature LH surge on hCG day were included. This study population was aged 27 to 43 years old and had visited Cheil General Hospital between January 2003 and January 2012. A premature LH surge was defined as LH levels above 10 mIU/mL or an increase of more than 2.5 times above the basal level on hCG day. We classified this study population into 3 groups: 1) no LH surge as controls (group A, 143 cycles, 126 patients), 2) premature LH surge with timed OPU 36-hour after hCG injection (group B, 129 cycles, 109 patients), and 3) premature LH surge with earlier OPU within 36-hour after hCG injection (group C, 35 cycles, 31 patients). The control group consisted of tubal factor infertility patients. Polycystic ovary patients were excluded. IVF outcomes, such as the number of retrieved oocytes, maturation rate, fertilization rate, clinical pregnancy rate, ongoing pregnancy rate, and miscarriage rate were compared among the 3 groups.
2. IVF protocol
On day 2 to 3 of the menstrual cycle, pelvic cavity abnormalities including ovarian tumors were checked through a pelvic ultrasound. The patients underwent IVF treatment using a flexible GnRH antagonist protocol and minimal stimulation protocol. In the flexible GnRH antagonist protocol, a daily 0.25-mg subcutaneous injection of cetrorelix acetate (Cetrotide, Serono, Darmstadt, Switzerland) or ganirelix acetate (Orgalutran, Schering-plough, Whitehouse Station, NJ, USA) was administered when the follicles were more than 12 to 14 mm in diameter or serum E2 concentrations were greater than 200 to 400 pg/mL. In the case of minimal stimulation, the patients were administered clomiphene citrate (2T, 100 mg/day) on menstrual cycle day 3 to 7. Then 150 to 225 IU of gonadotrophin (recombinant FSH, Gonal F, Serono, Geneva, Switzerland) was added according to the age of the patient and the ovarian reserve. The ovarian response to stimulation was monitored through the ultrasonographic size of the follicles and serum E2 levels. When a dominant follicle >18 mm in diameter was detected by ultrasonography, the serum LH and E2 levels were checked in the morning and reported in the afternoon. 250 µg choriogonadotropin-alfa (Ovidrel, Serono, Roma, Italy) was administered during the night. In group A and group B, transvaginal ultrasound-guided oocyte retrieval was performed 36 hours later after choriogonadotropin alfa injection. In group C, transvaginal ultrasound-guided oocyte retrieval was performed within 36 hours (generally about 24 hours) after choriogonadotropin alfa injection. We performed IVF or ICSI with either ejaculated sperm or surgically retrieved sperm. The embryos were transferred into the uterine cavity on day 2 to 5 after oocyte retrieval. Pregnancy was determined by a serial serum β-hCG level of >5 mIL/mL at 12 days after the oocyte retrieval. Clinical pregnancy was defined as the presence of a gestational sac by ultrasonography at approximately 5 weeks of pregnancy.
3. Statistical analysis
All analyses were performed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). One-way analysis of variance and Pearson's correlation analysis were used for the statistical analysis. Each variable is presented as mean±SD. A p<0.05 was considered statistically significant.
Results
In the earlier OPU group C, the age was older (38.2±5.4 vs. 36.8±4.9 vs. 36.2±4.2, [yr], p=0.012) and body mass index was higher (24.1±2.1 vs. 20.9±2.1 vs. 22.1±2.9, [kg/m2], p<0.001) than the timed OPU group B and control group A, respectively. The serum E2 values on hCG day were lower (415±354 pg/mL vs. 556±552 pg/mL, not significant [NS]) and the serum LH levels on hCG day were significantly higher (30.3±15.9 mIU/mL vs. 22.7±14.9 mIU/mL, p=0.04) in the earlier OPU group C than the timed OPU group B, respectively (Table 1).
4.9%, 31.7%, and 51.4% cycles had no oocytes in groups A, B, and C, respectively. The rates of totally cancelled cycles (OPU cancellation, no oocytes, or no embryos transferrable) were 15.4%, 65.9%, and 74.3% in the 3 groups. The number of retrieved oocytes did not differ between group B and group C (1.7±2.5 vs. 1.2±1.6, NS). There was no difference in maturation rates (83.9±23.2 vs. 79.7±35.1 vs. 60.7±43.2, [%], NS) among the three groups. However, the fertilization rate (73.2±38.9% vs. 47.8±42.9%, p=0.024) was significantly lower in the earlier OPU group than the timed OPU group, respectively (Table 2).
The clinical pregnancy rates and miscarriage rates were significantly higher in the earlier OPU group C than in the timed OPU group B (44.4% vs. 9.1%, p=0.021; 22% vs. 0%, p=0.026). There was no correlation between the LH surge levels and the pregnancy rates (data not shown). However, the ongoing pregnancy rate was higher but was not significantly different in the earlier OPU group C than the timed OPU group B (22% vs. 9.1%, NS) (Table 3).
Discussion
In the past, IVF with controlled ovarian hyperstimulation was disturbed by a 20% occurrence rate of a premature LH surge, which may be responsible for early luteinization and follicular atresia [15]. However, the GnRH antagonist protocol decreased the premature LH surge rate. Nevertheless, the protocol does not completely prevent the occurrence of a premature LH surge [16-19]. The premature LH surge is defined as a serum LH level of ≥10 mIU/mL [9-11]. This is known to reduce pregnancy rates in the case of a premature LH surge during COH, and few oocytes can be retrieved from poor responders [10,12,13]. Furthermore, no clear-cut threshold has been defined for cancelling an IVF cycle when a premature LH surge occurs [14].
Generally, the appropriate time for OPU is 34 to 36 hours after hCG injection. When physicians find a greatly elevated serum LH level on hCG day in an IVF cycle, they always worry about whether this surge is true or not, and whether the surge is an ascending limb or a descending limb. If this surge represents a descending limb, the scheduled OPU would be too late to obtain the oocyte. On the other hand, if this surge represents the ascending limb, an earlier OPU will not provide sufficient time for the oocytes to mature. To our knowledge, no previous study has evaluated the effectiveness of earlier OPU.
In our study, the premature LH surge groups were almost all poor responders. In these groups, the earlier OPU group showed much older and much higher LH levels on hCG day than in the timed OPU group. The earlier OPU group may be evaluated more and treated more carefully because of the higher risk during a premature LH surge. The earlier OPU group showed high basal FSH levels and low E2 levels on hCG day, low maturation rates, statistically significantly low fertilization rates, and higher OPU failure rates. Earlier OPU may be ineffective in terms of avoiding cycle cancellation. However, our data showed significantly higher pregnancy rates in earlier OPU than in timed OPU. We were not able to identify any reason for this, and there was no correlation between the LH surge levels and pregnancy rates. However, an earlier OPU may be effective in giving older infertile women a chance of achieving pregnancy, but our study population was too small to determine this. A larger prospective study will be required to clarify this point.
The limitations of this study are that our data did not include P4 levels on hCG day to define premature luteinization and included a relatively small population in the earlier OPU group.
In conclusion, earlier ovum pick up may not be effective for reducing the risk of cycle cancellation in patients with a premature LH surge on hCG day. A larger scale study will be required to reveal the effectiveness of earlier ovum retrieval in the case of a premature LH surge.
Notes
No potential conflict of interest relevant to this article was reported.