Rhox in mammalian reproduction and development
Article information
Abstract
Homeobox genes play essential roles in embryonic development and reproduction. Recently, a large cluster of homeobox genes, reproductive homeobox genes on the X chromosome (Rhox) genes, was discovered as three gene clusters, α, β, and γ in mice. It was found that Rhox genes were selectively expressed in reproduction-associated tissues, such as those of the testes, epididymis, ovaries, and placenta. Hence, it was proposed that Rhox genes are important for regulating various reproductive features, especially gametogenesis in male as well as in female mammals. It was first determined that 12 Rhox genes are clustered into α (Rhox1-4), β (Rhox5-9), and γ (Rhox10-12) subclusters, and recently Rhox13 has also been found. At present, 33 Rhox genes have been identified in the mouse genome, 11 in the rat, and three in the human. Rhox genes are also responsible for embryonic development, with considerable amounts of Rhox expression in trophoblasts, placenta tissue, embryonic stem cells, and primordial germ cells. In this article we summarized the current understanding of Rhox family genes involved in reproduction and embryonic development and elucidated a previously unreported cell-specific expression in ovarian cells.
Introduction
Approximately 200 homeobox genes have been identified in rodents and one third of them are expressed in the gonads. The homeobox is a sequence that encodes transcription factors containing the DNA binding motif or "homeodomain" composed of 60 amino acids. Homeodomain-containing transcription factors regulate diverse developmental and physiological events.
The best-known homeobox gene family is the Hox family. The Hox gene cluster was first identified by Lewis [1] in Drosophila melanogaster approximately 30 years ago. It is a group of genes that control the body plan development of the embryo along the head-tail body axis. The main characteristics of the Hox family are that it encodes transcription factors containing the homeobox and more particularly, in mammals, it displays colinearity, in that the organization of Hox genes on the chromosome is the same order as their expression along the heal-tail body axis during embryonic development [2]. This temporal and spatial expression feature, that is, the colinearity of these genes, is related to the proper regulation of the development of their target tissues.
In 2005, approximately 20 years after the discovery of the homeobox, MacLean et al. [3] reported the discovery and characterization of new homeobox genes. These new homeobox genes, reproductive homeobox genes on the X chromosome (Rhox) are expressed selectively in male and female reproductive tissues in a cell type- and region-specific manner and play pivotal roles in embryonic development and adult reproduction [4,5]. In this review, we summarize the expression and roles of this new family of transcription factors Rhox in relation to reproduction and development.
Rhox family genes
1. Genomic structure of Rhox
It was first determined that 12 Rhox genes are clustered into α (Rhox1-4), β (Rhox5-9), and γ (Rhox10-12) subclusters, and more recently, Rhox13 has been found. At present, 33 mouse genes have been found in this region and it comprises the largest homeobox gene cluster known in the mouse genome [5]; in the rat, 11 such genes have been identified, and three in the human. Rhox genes are extended to the large genomic region within an around 0.7 Mb segment in the A2 region of the mouse X chromosome [3]. The Rhox genes in the cluster have been classified by number according to their positional order on the X chromosome; the closest one to the centromere was named Rhox1, and the farthest, Rhox12.
The mouse Rhox gene family contains α, β, and γ subclusters [5]. The subcluster was originally defined as having four genes (Rhox1-4), but subsequent scrutiny of the X chromosome by genomic mapping and sequencing led researchers to understand that tandem replications of Rhox2, Rhox3, and Rhox4 make a total of 23 genes in the 350 kb A2 genomic region (position 29780 K to 30100 K) between Rhox1 and Rhox5. The replicated copies, namely paralogs, of each of these three genes are almost identical in sequence (more than 95% similarity). The α subcluster consists of eight paralogs of Rhox2 and Rhox3 and seven paralogs of Rhox4. At first, Rhox4 was called Ehox, since it was initially identified in embryonic stem cells [6]. The β subcluster has five genes (Rhox5-9), and four of them were previously known by other names, specifically, Pem (Rhox5), Psx1 (Rhox6), Tox (Rhox8), and Psx2 or Gpbox (Rhox9). Finally, the γ subcluster is identified as four genes (Rhox10-13) [3,7,8], and Rhox13 was discovered most recently [9].
The rat Rhox gene clusters are smaller than those in the mouse because it has only a single copy of α subcluster paralogs without Rhox1. Another difference from the mouse Rhox gene cluster is that the rat genome does not contain one of the β cluster genes, Rhox6, or Rhox13 [10]. The dispositions of Rhox genes in the mouse and the rat are almost the same, suggesting the gene arrangements occurred by the mouse/rat split [5].
The human RHOX gene cluster is much smaller than those of rodents. Only two human RHOX orthologs have been detected on the region corresponding to the rodent X chromosome: RHOXF1 (PEPP1/OTEX) and RHOXF2 (PEPP2) [3]. Recently, Niu et al. [11] reported that: 1) 11 nonhuman primate species have one RHOXF2 copy, 2) humans and 4 Old World monkey species have 2 copies, RHOXF2 and RHOXF2B, and 3) chimpanzees have at least 6 copies of RHOXF2.
2. Expression of RHOX protein
The homeobox genes encode transcription factors containing a DNA-binding domain, namely the homeodomain, of 60 amino acids that has three α-helices. It is an important concept that homeodomain proteins bind not only to specific sequences on DNA, but also to specific proteins or RNAs [12,13]. This demonstrates the pivotal role of homeodomain transcription factors in various biological processes. Most mouse RHOX proteins have a very similar length and their homeodomains are located at the conserved position. Figure 1 depicts a schematic diagram of the RHOX protein products based on the data from the MacLean group [3] and Geyer and Eddy [9]. It shows that the RHOX proteins are similar in size and contain a single homeodomain near the C-terminus.

Schematic diagram of the reproductive homeobox genes on the X chromosome (Rhox) proteins. RHOX protein family members have a similar length, with a single homeodomain (pink box) near the C terminus. N, N-terminus; C, C-terminus.
Homeobox gene product RHOX5 has been suggested to be a transcription factor that regulates a set of genes [3,4]. Prosaposin (PSAP), menin (MEN1), inhibitor of MyoD family (I-MFA), and cell division cycle 37 (CDC37) have been identified as interacting partners of RHOX5 [5]. The Rhox8 gene encodes the protein with a length of 293 amino acids, and its homeodomain is close to the carboxy terminal region. Exceptionally, RHOX8 is highly glutamic acid rich and has two long glutamic acid repeats (25 and 26 amino acids, respectively). This makes RHOX8 a very acidic protein (pI=3.75) due to 35% of the total protein being composed of glutamic acids [14]. The newly discovered gene, Rhox13, encodes a 232-amino acid protein. It was predicted to be a 25.3 kDa protein, but the rabbit antisera against the N-terminus of RHOX13 RHOX13 detected band at around 45 kDa from adult mouse testis homogenates using Western blot analysis [9]. To clarify the possible explanations for this size difference, Geyer and Eddy [9] generated a recombinant RHOX13 fusion protein with an N-terminal hexahistidine-maltose binding protein (His-MBP) tag, and found that the primary sequence of RHOX13 was responsible for its aberrant migration. The regulation of RHOX protein expression and its modification is not yet well understood. In addition, the function of RHOX as a transcription factor, and whether it acts as an activator or repressor, has not yet been determined.
Characteristics of Rhox gene expression
1. Cell type-specific expression of Rhox
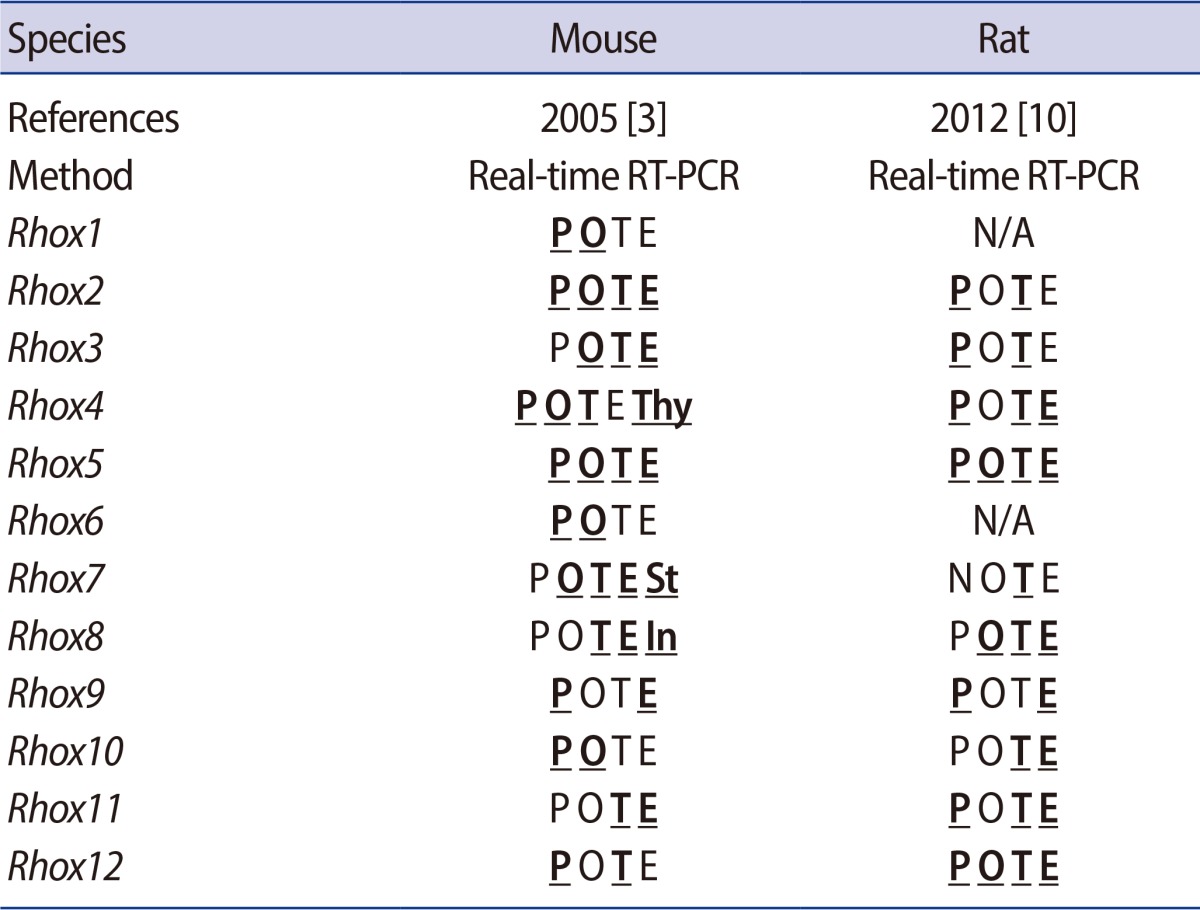
Rhox genes are selectively expressed in male and female reproductive tissues: in the testes, epididymis, ovaries, and placenta. MacLean et al. [3,10] examined the pattern of Rhox gene expression in the mouse and rat in 2005 and 2012, respectively, by using real-time reverse transcription PCR (RT-PCR) analysis. Tissue-specific types of Rhox expression and their relative expression levels are summarized in Table 1. Most Rhox family members are expressed in reproductive-associated tissues. MacLean et al. [3] confirmed by Northern blot analysis the major expression of Rhox1 in the ovary and Rhox3, 8, and 11 in the testis; while Rhox2, 4, 5, 6, 9, 10, and 12 were found in the placenta. Tissue expression of Rhox13, discovered by Geyer and Eddy [9] in 2008, could not be included in their expression studies due to lack of knowledge of this gene in the mouse in 2005 and no ortholog yet found in the rat.
Additionally, mouse Rhox4, 7, and 8 are expressed in non-reproductive organs, such as the thymus, stomach, and intestine, respectively (Table 1). Daggag et al. [15] analyzed the expression of nine murine Rhox genes during mouse embryonic gonad development by means of real-time RT-PCR, and found the remarkable expression of Rhox8 at the somatic counterpart of the embryonic gonad between E12.5 and E15.5.
Some of the Rhox genes are sexually biased; Rhox6 and Rhox9 are predominantly expressed in embryonic female germ cells, whereas Rhox10 is only present in embryonic male germ cells. Transcripts of Rhox3, Rhox8, and Rhox11 are found in the adult mouse testes, while Rhox10 is detectable at the fetal testes. Expressions of Rhox1, Rhox6, and Rhox7 mRNA are detected in the fetal ovaries, while Rhox2a, Rhox4a, Rhox5, and Rhox9 are detectable in both the fetal ovaries and testes [15]. Song and colleagues analyzed human RHOX gene and protein expression patterns in 11 fetal and 8 adult tissues. They found that RHOXF1 and RHOXF2/B mRNA are highly expressed in the human testes but marginally in the ovaries and non-reproductive tissues. In the testes, early stage germ cells (spermatogonia and early spermatocytes) express RHOXF2/2B, while later stage germ cells (pachytene spermatocytes and round spermatids) express RHOXF1. In the ovaries, RHOXF1 and RHOXF2/2B proteins are exclusively found in the oocytes rather than the whole ovary, where low levels of RHOX mRNA expressed [16].
2. Temporal and quantitative colinearity of Rhox expression
Expressions of Rhox genes show dynamic changes depending on developmental status. Expression of the α subcluster genes exhibit temporally and quantitatively unidirectional expression patterns, namely colinearity, corresponding to their position within the subcluster. For instance, Rhox1, the closest gene to the centromere of the α subcluster, is expressed first between postnatal days 7 and 12; after that, its expression was found to have disappear in the mouse testes. Around postnatal day 12, the expression of the next gene, Rhox2, is at its peak. The expression of Rhox3 and 4 reach a peak between postnatal days 20 and 22. This phenomenon has been termed "colinearity" in temporal or quantitative expression [3].
In the case of β subcluster genes, colinearity in temporal expression is not observed, but Rhox5, 7, and 8 do show a quantitative unidirectional expression. Interestingly, the expression of Rhox6 or Rhox9 is completely silent in the testis [3]. Similar to the α subcluster, γ subcluster genes show both temporal and quantitative colinearity. First, Rhox10 exhibits its peak expression around postnatal day 12, and then Rhox11 and 12 initiate their expression at the time that Rhox10 expression begins to decrease around day 18. In summary, subclusters α and γ showed both temporal and quantitative colinearity, while subcluster β showed only quantitative colinearity [3].
Expression and function of the Rhox family
1. Rhox in the male reproductive system
The selective expression of the Rhox genes in reproductive-associated tissues suggests its important regulatory roles in reproductive features, such as in gametogenesis and fertility. Although little is known about which cells in adult reproductive organs express individual Rhox genes, relatively substantial data have been reported about Rhox5, and it is the only mammalian homeobox gene known to play a role in spermatogenesis [3,4,17]. However, conflicting results in male fertility have been reported. Male subfertility has been reported in Rhox5-null mice, characterized by increased germ cell apoptosis, reduced sperm number and sperm motility, and small litter size [4]. These findings conflictied with previously reported data that showed no obvious defects in Pem homeobox gene-deficient mice (Pem was the original name for Rhox5) [18].
Expression of Rhox genes in the Sertoli cells that nourish and regulate diverse characteristics of male germ cells, such as proliferation and differentiation, imply the sex hormone regulation of Rhox expression [19-21]. It has been reported that androgen induced Rhox5 expression and that modulated the expression of androgen receptor (AR)-regulated genes such as phospholipid transfer protein (Pltp), transmembrane protein 47 (Tmem47), Tmem176b, ganglioside-induced differentiation-associated protein1 (Gdap1), and frizzled homolog 2 (Fzd2) in Rhox5-positive 15P-1 Sertoli cell clones [21]. In that study, they identified genes downstream of Rhox5 in the testis, such as CD24a antigen (Cd24a), Kruppel-like factor 9 (Klf9), carboxypeptidase 1 (Cpxm1), Tmem176a, and Tmem176b.
By using a gain-of-function approach and microarray analysis in 15P-1 cells, Hu et al. [4,17] found the list of Rhox5-regulated genes. Rhox5 negatively regulates the transcription of Unc5c proapoptotic receptor with tumor suppressor activity in Sertoli cells both in vivo and in vitro, and the Unc5c 5'-UTR has Rhox5-responsive cis-regulatory elements. Similar activity of Rhox2 and Rhox3 to Rhox5 suggests the possible redundancy in Sertoli cells. This Unc5c repression by mouse Rhox2 and Rhox3 is elicited by human RHOXF2.
The epididymis is a highly segmented organ and is divided into three regions: the caput (proximal), corpus, and cauda (distal region). In general, mouse Rhox genes are abundant in the caput region and show gradient expression in the direction from the caput to the cauda [8]. Rhox5-null mice display an altered expression of the majority of the other Rhox genes, mostly lower in the caput, with only Rhox6 higher in both the caput and cauda of the epididymis in Rhox5-null mice [8]. These results imply a compensation mechanism indicating that Rhox genes could reciprocally interact with each other and the nearest gene, Rhox6, could be an alternative in the absence of Rhox5. In addition, these results show that Rhox5 is a master regulator of many other members of the Rhox family of genes in the murine epididymis.
Transcription of Rhox5 mRNA is regulated by two promoters, a distal promoter (Pd) and a proximal promoter (Pp), which are each independently regulated [5,22]. While the Pp is restricted to somatic cells in the testes and epididymis, the Pd is expressed in the early embryo and somatic cells in adult female reproductive tissues, specifically the ovary and placenta, and is co-expressed within the Pp in the testes [23]. In addition, the Pd is widely found in many cells such as primary granulosa cells, mesenchymal stem cells, and tumor cells originateing from various cells or tissue lineages [5]. Rhox5 transcription is regulated not only by hormonal regulation but also the site-specific methylation of cytosine and guanine in the Pp promoter. Androgen and AR act cooperatively on the AR-response element, and AR is recruited to the Pp in a region-specific and time-dependent manner. GATA-binding sites are also crucial for Pp-dependent Rhox5 mRNA expression in the epididymis. These androgen and androgen receptor system, and GATA factors collaborate to regulate the expression of Rhox5 mRNA in the epididymis [24].
Rhox10, a member of mouse γ subcluster Rhox genes, is abundantly expressed in immature male germ cells, such as fetal gonocytes, spermatogonial, and spermatocytes. Production of Rhox10 mRNA of spermatogonia cells is dramatically induced by treatment with retinoic acid, wich provides an extrinsic cue to germ cells to enter meiosis [25]. When we measured mRNA expression in the testes and dissected cells from the female gonad, including oocytes, cumulus cells, and granulosa cells, we also found that Rhox10 mRNA was the most abundantly expressed type of Rhox mRNA in the ovaries as well as the testes (Figure 2).
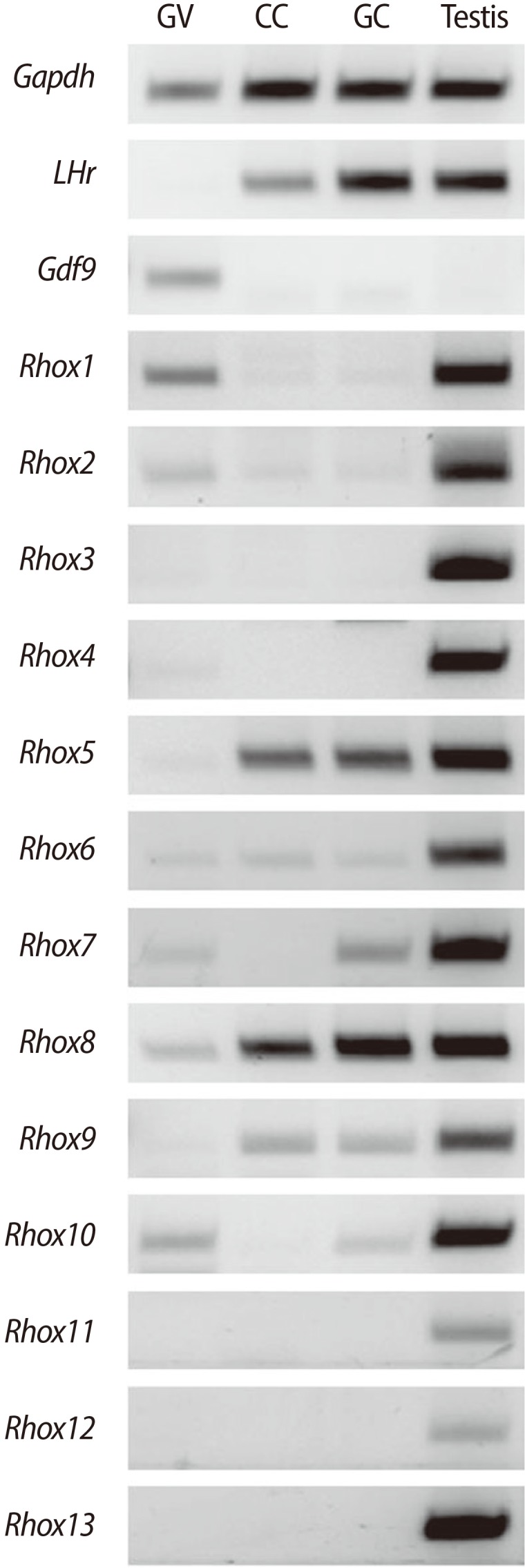
Expression of reproductive homeobox genes on the X chromosome (Rhox) family members in dissected mouse ovarian tissues, specifically oocytes, cumulus cells, granulosa cells, and testes. Gdf9 and LHr were used as markers for oocytes and granulosa cells, respectively. Gapdh was used as an internal control. In the case of the oocytes, PCR was carried out by using cDNAs equivalent to a single-oocyte. These pictures are representative of the experiments, which were repeated five times. GV, denuded germinal vesicle oocytes; CC, cumulus cells; GC, granulosa cells.
Rhox13 mRNA and protein expression have been detected in the ovaries from embryonic day 13.5 (E13.5), but RHOX13 protein expression is suppressed until postnatal day 3 in male mice [26]. Geyer et al. [26], who first found the Rhox13 gene and its expression in germ cells in the fetal testis and ovary, reported that NANOS2 localized in P-bodies has the activity of deadenylase and degradation of specific mRNA. The RHOX13 protein was detectable in NANOS2-null mice at E15.5 but not in heterozygous mice. This suggests that the RHOX13 protein is regulated by an RNA-dependent mechanism.
RHOXF1 and RHOXF2/2B are the most abundant in the human testis. Early-stage germ cells (spermatogonia and early spermatocytes) express RHOXF2/2B, while later-stage germ cells (pachytene spermatocytes and round spermatids) express RHOXF1. RHOXF1 and RHOXF2/2B mRNA expression increases gradually with the gestational days of the fetal testes [16]. In summary, the Rhox genes of the male reproductive system are tightly regulated in a temporal, spatial, and hormone-dependent manner.
2. Rhox in the female reproductive system
Rhox genes also play key roles in the female reproduction system. MacLean et al. [3] previously showed that adult mouse ovaries express significant amounts of Rhox1, Rhox2, Rhox5, and Rhox7 by real-time RT-PCR and Rhox1 using northern blot analysis. The same authors found a slightly different expression pattern in the rat ovaries, in which Rhox expression was relatively lower [10]. Transcripts of Rhox1, 2a, 4a, 5, 6, 7, and Rhox9 are detectable in female germ cells between E12.5 and E15.5 [15].
We measured the expression of all 13 members of Rhox family genes by RT-PCR in germinal vesicle (GV) oocytes, cumulus cells, and granulosa cells dissected out from 21-day-old mice 48 hours after equine chorionic gonadotropin (eCG) stimulation (Figure 2). All 13 members were expressed in the testes, whereas, interestingly, only some of them were expressed in the oocytes and follicular cells. Expression of Rhox3, 4, 11, 12, and 13 was not detectable in the ovarian cells. Only expression of Rhox1 and 2 was detectable in the adult germ cells, but not in the follicular cells, whereas Rhox5 and 9 were detectable in the follicular cells but were only very slightly expressed in the oocytes. Facinatingly, Rhox7 and 10 were detectable in the oocytes and granulosa cells but not in the cumulus cells, since the cumulus cells are a cluster of granulosa cells closely surrounging the oocyte. It strongly suggests that the reciprocal interactions and powerful effects between oocyte and cumulus cells on their each others' gene expression. Finally, only Rhox5 and 9 were constitutively expressed in ovarian cells. As mentioned earlier, the Rhox promoter, especially the distal promoter Pd was substantially transcribed in female reproductive tissues in the ovary and placenta; therefore, based on our results in Figure 2, it would be a good model for studying the tissue- and stage-specific and aberrant regulation of Rhox gene expression.
Promoter Pd for Pem (Rhox5) is transcriptionally regulated by the Ets transcription factors, Gabp and Elf1, and the ubiquitous zinc finger transcription factors, Sp1 and Sp3, in normal granulosa cells [27]. The Ets family transcription factors are closely related to diverse tumor cell types for tumor cell growth, invasion, and metastasis, and to the proper control of proliferation and differentiation of normal cells. Adult human ovaries express a significant amount of RHOXF1 and RHOXF2/2B proteins primarily in oocytes, despite low levels of RHOX mRNA in the whole ovary [16]. However, little is known about their function in the human reproductive organs.
The gene expression of Rhox2, 5, 6, and 8 is affected by the ovulatory cycle, but their downstream genes are unknown in the female reproductive tissues [28]. Researchers have observed that Rhox8 displayed a unique expression pattern with the progesterone response element within the Rhox8 promoter in granulosa cells. In addition, they found that Rhox8 mRNA expression in Rhox5-null mice was reduced after hCG administration but recovered with follicular development. Rhox8 exhibited normal stimulation by eCG but failed to reach its peak mRNA level at 8 hours post-hCG, as found in wild-type ovaries, in progesterone receptor knockout (PRKO) mice. Based on these results, they conclude that Rhox8 transcription is dependent on Rhox5 during early folliculogenesis and on progesterone during the periovulatory window [28]. The same researchers also found that the mouse Rhox α and β subcluster genes are induced by FSH and LH, whereas Rhox7 and the γ subcluster genes remained silent in superovulation.
3. Rhox in stem cells and embryonic development
In addition to the roles of Rhox genes in reproductive biology, Rhox genes are also involved in the differentiation and/or regulation of several types of stem cells, including embryonic stem cells, primordial germ cells, and trophoblast stem cells. Rhox4 (Ehox) expression is found in embryonic stem cells and in vitro differentiation of embryonic stem cells with transient knocked down of Rhox4 without leukemia inhibitory factor impairs hematopoietic, endothelial, and cardiac differentiation. Thus Rhox4 is considered essential for the earliest stages of murine embryonic stem cell differentiation [6]. As mentioned earlier, at present, 33 Rhox genes have been found in mice, 11 in rats, and three in humans. Jackson et al. [7] postulated that this unique clustering of Rhox genes among mice, rats, and humans may explain species differences in embryonic stem cell derivation and maintenance. They found important comparable functions of two Rhox genes within the duplicated region of the cluster of Rhox2 to Rhox4, in regulating the initial stage of embryonic stem cell differentiation and maintenance. It has been reported that Rhox4 is also expressed in trophoblast stem cells, supporting a role for Rhox4 in the placental stem cells in the developing placenta [3,29].
It has been suggested that Rhox5 plays a vital role in the differentiation of embryonic stem cells. Rhox genes are abundant in E9.5 trophoblasts and Rhox5, which is localized in the X chromosome, is expressed predominantly in female mouse blastocysts rather than male blastocysts [30]. Embryonic stem cells with constitutively expressing Rhox5 (Pem) are not differentiated into the primitive endoderm nor embryonic ectoderm, but embryonic stem cells with constitutively expressing Rhox5 (Pem) developed into the teratoma containing only undifferentiated embryonic carcinoma-like cells. This differentiation inhibition of embryonic stem cells with forced expression of Rhox5 in vitro and in vivo suggests a role for Rhox5 in the transition between undifferentiated and differentiated cells in the early mouse embryo [31].
All Rhox genes, except Rhox8, showed germ cell-specific expression in the embryonic testes and ovaries [15]. Rhox8 was found to be expressed within the somatic compartments. In addition, Rhox expression in embryonic gonads showed dynamic and sexually dimorphic patterns with a tendency toward higher expression in female germ cells, with the single exception of Rhox10, which showed exclusive expression in male embryonic germ cells. These results imply an important developmental role for Rhox genes in embryonic gonads [15].
Meanwhile, Rhox6 is abundantly expressed in the placenta and the post-migratory primordial germ cells. Continuous knock-down of Rhox6 disturbed the primordial germ cell differentiation process, implying that Rhox6 is necessary in the regulation of germ line differentiation [32]. Rhox9 is 91% identical to Rhox6 in the protein-coding nucleotide sequence and has been identified in the primordial germ cells, placenta, female embryonic stem cells, and in the ovary [33]. The histone demethylase Lysine (K)-specific demethylase 6A (KDM6A, also known as UTX; ubiquitously transcribed tetratricopeptide repeat, X chromosome), that removes H3K27me3 from HOX genes to restore their activity, is encoded by an X-linked gene that escapes X inactivation in somatic tissues of the mouse and human [34]. This suggests that KDM6A may be important for female-specific junctions [35]. Berletch et al. [33] found that Rhox6, Rhox9, and Kdm6a are expressed in a sexually dimorphic manner. Expression of Rhox6 and Rhox9 are significantly higher in undifferentiated female embryonic stem cells, and this female bias was due to KDM6A, which occupied the 5' end of Rhox6 and Rhox9 to regulate their expression. It has been found that Rhox6, Rhox9, and Kdm6a are highly expressed in the ovary, showing the consistent paternal imprinting of these genes previously reported in the placenta and embryonic stem cells [36].
Future directions
The Rhox family of genes are recently identified homeobox genes, and similar to other homeobox genes, these genes are selectively expressed in embryonic and adult reproductive tissues and stem cells. In light of their spatially and temporally limited expression pattern, their functions in reproduction and development have been proposed and confirmed one by one.
The Rhox genes have been studied for the last two decades; however, much remains to be clarified regarding the biological significance and function of individual paralogs, as well as their regulation. It is evident so far that Rhox expression and regulation are important during embryogenesis and gametogenesis as well as in the reproductive physiology of adults. The regulatory mechanism of Rhox genes and their roles in reproduction and stem cell biology should be scrutinized in the future.
Notes
This research was supported by the Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0093821).
No potential conflict of interest relevant to this article was reported.